Page 1 of 1
15.17
Posted: Mon Feb 26, 2018 11:35 pm
by Mia Navarro 1D
Why, when solving for the rate, is [B] squared?
Re: 15.17
Posted: Tue Feb 27, 2018 1:25 am
by Caroline LaPlaca
No, cause its not like the K concentration thing.
Re: 15.17
Posted: Tue Feb 27, 2018 10:33 am
by Johann Park 2B
With respect to [B], the reaction is second-order. You use the different values for the rates and concentrations to solve for the exponents (x, y, z) on the concentrations.
Re: 15.17
Posted: Tue Feb 27, 2018 5:36 pm
by OliviaShearin2E
Johann is right but to be clearer, first order in B means that the rate is proportional to
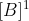
, second order in B means that the rate is proportional to
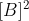
. and third order in B means that the rate is proportional to
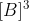
.
Re: 15.17
Posted: Tue Feb 27, 2018 10:03 pm
by Sandhya Rajkumar 1C
The reaction is second order with respect to B because when the amount of B is doubled (increased by a factor of 2), the rate increases by a factor of 4, so instead of it being a 1:1 ratio, which would make it first order, it's a 1:2 ratio, which makes it second order.