15.23 b
Moderators: Chem_Mod, Chem_Admin
-
Andrea Grigsby 1I
- Posts: 60
- Joined: Fri Sep 29, 2017 7:03 am
Re: 15.23 b
I used the equation 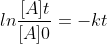 and got the same answer as the solutions manual. I think the two equations are equivalent because of the negative sign I used in my answer, when transferred to the other side, would flip the two concentrations.
and got the same answer as the solutions manual. I think the two equations are equivalent because of the negative sign I used in my answer, when transferred to the other side, would flip the two concentrations.
-
David Zhou 1L
- Posts: 61
- Joined: Fri Sep 29, 2017 7:04 am
Re: 15.23 b
It's just algebraic manipulation; flipping the numerator and denominator will give you the negative log value.
-
Jenny Cheng 2K
- Posts: 30
- Joined: Fri Sep 29, 2017 7:05 am
- Been upvoted: 1 time
Re: 15.23 b
If you make both sides of the equation ln([A]t/[A]0) = -kt negative, you will have: ln([A]0/[A]t) = kt. Making the natural log negative flips the content because:
ln([A]t/[A]0) = ln[A]t - ln[A]0
ln([A]t/[A]0) = ln[A]t - ln[A]0
Who is online
Users browsing this forum: No registered users and 9 guests

