Fractional Rate Law [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
RuchaDeshpande1L
- Posts: 60
- Joined: Sat Jul 22, 2017 3:00 am
- Been upvoted: 1 time
Fractional Rate Law
If we are given a rate law that involves a fraction of concentrations, such as 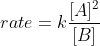 , do we still add the exponent values to find the overall order of the reaction? Like in this case, would the overall order be 2 + (-1) = 1? Thanks for clarifying!
, do we still add the exponent values to find the overall order of the reaction? Like in this case, would the overall order be 2 + (-1) = 1? Thanks for clarifying!
-
Adam Enomoto 1L
- Posts: 53
- Joined: Fri Sep 29, 2017 7:03 am
Re: Fractional Rate Law
https://chemistry.stackexchange.com/que ... -mechanism
This website might help with it. On page 621 the textbook talks about fractional order reactions. I'm not entirely sure, but if the fractional order is in the denominator, it means that the reactions slows down as the concentration of it increases.
This website might help with it. On page 621 the textbook talks about fractional order reactions. I'm not entirely sure, but if the fractional order is in the denominator, it means that the reactions slows down as the concentration of it increases.
-
Rachel Lu_dis1H
- Posts: 66
- Joined: Fri Sep 29, 2017 7:06 am
- Been upvoted: 1 time
-
Deborah Cheng 1F
- Posts: 50
- Joined: Thu Jul 13, 2017 3:00 am
Re: Fractional Rate Law
Yes I believe you are both correct, you would just calculate the order according to normal exponent addition/subtraction rules.
-
Luis De La Cruz 1H
- Posts: 37
- Joined: Fri Sep 25, 2015 3:00 am
Re: Fractional Rate Law [ENDORSED]
Yes, that is exactly how you would be able to calculate the overall order of the reaction, and you should also keep in mind that because [B] is at the bottom, that will affect the units of K as well. This is basically like the first question we got on test 3 for kinetics.
Who is online
Users browsing this forum: No registered users and 11 guests

