n
Moderators: Chem_Mod, Chem_Admin
-
Hena Sihota 1L
- Posts: 51
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 2 times
-
Andres Reynoso 1J
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Re: n
I believe in lecture Friday, Professor Lavelle also used 'n' to represent the order of the reactant in the "rate law" ( 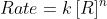 ) if that is what you are referring to when you say kinetics.
) if that is what you are referring to when you say kinetics.
-
Michelle Lee 2E
- Posts: 64
- Joined: Thu Jul 27, 2017 3:01 am
Re: n
n refers to the order of the reactants. But there could also be more orders of reactants which can be denoted by other variables. Since in the recent equations we've been using, we've been dealing with concentrations which is moles per liter (like moles are already incorporated into the equation through this means), we can safely assume that n would most likely mean order of reactants.
-
Matthew 1C
- Posts: 47
- Joined: Fri Sep 29, 2017 7:06 am
Re: n
Keliana Hui 2E wrote:What does order of reactants mean?
The order of the reactant is the value of n, which is the exponent of the concentration of the reactant in the rate law
-
Peri Bingham 1G
- Posts: 50
- Joined: Fri Sep 29, 2017 7:03 am
Re: n
The order of reactants is the sum of the powers that individual concentrations in a reaction's rate law are raised to.
-
Fatima_Iqbal_2E
- Posts: 31
- Joined: Fri Sep 29, 2017 7:06 am
- Been upvoted: 1 time
Re: n
'n' represents the order of the reactants in the rate law and gives insight into the reaction mechanism, you would see it as the exponent on the concentration of the reactants: 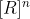 .
.
Return to “Method of Initial Rates (To Determine n and k)”
Who is online
Users browsing this forum: No registered users and 6 guests

