Page 1 of 1
k' vs kr
Posted: Mon Mar 09, 2020 12:34 am
by Jorja De Jesus 2C
What is the difference between k' and
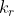
?
Re: k' vs kr
Posted: Mon Mar 09, 2020 12:44 am
by Nawal Dandachi 1G
K' is reverse reaction
Re: k' vs kr
Posted: Mon Mar 09, 2020 1:21 am
by Kevin Xu 4F
K' represents the placeholder K that is used in psuedo order reactions when the rate law is simplified to only include one species at reaction. For example, if the rate law of a reaction was k[A][B][C] and we wanted to isolate the changes affecting species A, then we could shorten and truncate the rate law into k'[A] where k' is equal to k[B][C]. This is so that it simplifies the equation and turns it into a psuedo first order rate law.
Re: k' vs kr
Posted: Mon Mar 09, 2020 9:02 am
by Christineg1G
k' is the rate of the reverse reaction. kr is the rate constant of a reaction that depicts the rate of a chemical reaction.
Re: k' vs kr
Posted: Mon Mar 09, 2020 11:39 am
by 805422680
K’ is the rate of the reverse reaction. kr is the rate constant of the reaction
Re: k' vs kr
Posted: Mon Mar 09, 2020 12:05 pm
by Hannah Romeo 1J
K’ is the rate of the reverse reaction which equals Kr
Re: k' vs kr
Posted: Mon Mar 09, 2020 5:03 pm
by Sam McNeill 1E
Additionally, kr/k prime=K, the equilibrium constant.
Re: k' vs kr
Posted: Mon Mar 09, 2020 5:24 pm
by BCaballero_4F
kr is the rate constant of the reaction, while k' is the rate of the reverse reaction, their relation can be shown as kr/k'=K
Re: k' vs kr
Posted: Tue Mar 10, 2020 7:36 pm
by Jainam Shah 4I
K' is the rate constant for the reverse reaction which is why k/k' is the equilibrium constant K
Re: k' vs kr
Posted: Wed Mar 11, 2020 12:09 am
by ShastaB4C
Is k’ and k prime have to be positive right? Like just because k’ is the reverse reaction doesn’t mean it has a negative sign
Re: k' vs kr
Posted: Wed Mar 11, 2020 12:17 am
by Adriana_4F
ShastaB4C wrote:Is k’ and k prime have to be positive right? Like just because k’ is the reverse reaction doesn’t mean it has a negative sign
Yes, it always has to be positive because the Arrhenius Equation is always positive.
Re: k' vs kr
Posted: Wed Mar 11, 2020 12:39 am
by Madeline Phan 1E
k' is the rate of the reverse reaction.
Re: k' vs kr
Posted: Wed Mar 11, 2020 7:37 am
by Ashley Tran 2I
to clarify, reverse rate means the reciprocal. k'=1/k
Re: k' vs kr
Posted: Wed Mar 11, 2020 7:50 am
by Max Madrzyk Dis 4G
k' is just for the reverse reaction and kr is for the forward reaction.
 ?
?