"The rate of the given reaction is 0.600 M/s.
A+4B⟶2C
What is the relative rate of change of each species in the reaction?"
What is the easiest method of solving for the relative rate of change? I get confused on how to do it.
Sapling Week 9/10 Question 1
Moderators: Chem_Mod, Chem_Admin
-
Tanya Bearson 2K
- Posts: 55
- Joined: Thu Dec 17, 2020 12:18 am
Re: Sapling Week 9/10 Question 1
You can use the equation: rate = (-1/coefficient of species)*(change in concentration of species/change in time) to find the relative rate of each species which is the (change in concentration of species/change in time) part of the equation. For example for A you would use the equation .600 M/s = (-1/1)*(delta[A]/delta t) to find that (delta[A]/delta t) = -.600 M/s
Last edited by Tanya Bearson 2K on Sun Mar 07, 2021 11:17 pm, edited 1 time in total.
-
Stacey Phan 2I
- Posts: 113
- Joined: Wed Sep 30, 2020 9:59 pm
- Been upvoted: 1 time
Re: Sapling Week 9/10 Question 1
I think you just multiply the rate by the coefficient. Reactants have negative rates since they are being consumed.
-
Melanie Lin 3E
- Posts: 99
- Joined: Wed Sep 30, 2020 9:38 pm
Re: Sapling Week 9/10 Question 1
Hi! I honestly suck at explaining but I'll do my best. I usually just remember its always (-1/a)*(d[A]/dt)=rate or any of the reactants (make sure to have that negative sign because they technically lose reactants as the forward reaction goes). As for products, it'll be (1/c)*(d[C]/dt)=rate (it'll be positive because we gain products). For example, (-1/b)*(d[B]/dt)=rate so d[B]/dt=(0.600 M/s)*(-4). Hope this helps!
-
Christine Ma 3L
- Posts: 102
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Sapling Week 9/10 Question 1
In general the rate of a reaction of the form 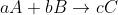 is equal to =
is equal to = 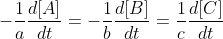 .
.
The solve for the relative rate of change of each reactant, you just multiply the overall reaction rate by its negative coefficient (since reactants are decreasing).
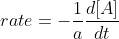
\times rate)
\times rate)
For the relative rate of change of a product, you just multiply the reaction rate by its coefficient.
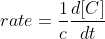
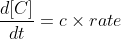
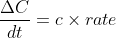
The solve for the relative rate of change of each reactant, you just multiply the overall reaction rate by its negative coefficient (since reactants are decreasing).
For the relative rate of change of a product, you just multiply the reaction rate by its coefficient.
-
Ven Chavez 2K
- Posts: 101
- Joined: Mon Feb 24, 2020 12:16 am
Re: Sapling Week 9/10 Question 1
The stoichiometric coefficients provide the information needed to solve the relative rate change. I think of it as 1A is consumed, 4B is consumed, and 2C is made. Therefore you multiply the rate of the reaction by how much each reactant is used and how much product is created. So you multiply the rate by 1 for A, multiply by 4 for B, and multiply by 2 for C. Since reactants are being consumed and their concentration decreases, the reactants have a negative rate. Since products are being formed and their concentration increases, the products have a positive rate.
-
SashaAnand2J
- Posts: 100
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Sapling Week 9/10 Question 1
Hi Sean!
The unique rates per product and reactant are based upon stoichiometric coefficients. The inverse of the stoichiometric coefficient multiplied by the change in concentration over time of a particular substance is equal to the overall rate of the reaction. This should also help you relate the reactants to the products! Hope that helps.
The unique rates per product and reactant are based upon stoichiometric coefficients. The inverse of the stoichiometric coefficient multiplied by the change in concentration over time of a particular substance is equal to the overall rate of the reaction. This should also help you relate the reactants to the products! Hope that helps.
-
Daniela Santana 2L
- Posts: 103
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Sapling Week 9/10 Question 1
Hi! I solved this question by looking at the reaction and taking note of the coefficients in front of each substance. I would essentially find the reciprocal of the coefficient and put that in instead of the coefficient thats given. For example, the 4 in 4B would become 1/4. I would rewrite the whole reaction this way and set it equal to the rate (.600). You need to find the value of which you can multiply by the coefficient for each substance and get .600. I hope this helped!
Re: Sapling Week 9/10 Question 1
all you have to do is multiply the rate they give you by the coefficient in the chemical equation! :)
Return to “Method of Initial Rates (To Determine n and k)”
Who is online
Users browsing this forum: No registered users and 5 guests

