Determine the average rate of change of B from t=0 s to t=292 s.
A⟶2B
Time (s) Concentration of A (M)
0 0.790
146 0.485
292 0.180
rate B= ? M/s
how would you approach solving this problem?
sapling #2
Moderators: Chem_Mod, Chem_Admin
-
Samantha Pedersen 2K
- Posts: 134
- Joined: Wed Sep 18, 2019 12:21 am
- Been upvoted: 9 times
Re: sapling #2
First, note that they are asking for the average rate of change of B. We learned that the average rate is equal to 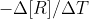 or
or 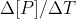 . Since we only have data for the reactant, we should use the first version of the average rate equation. This gives us -(final concentration of A - initial concentration of A) / (final time - initial time). This will give us the average rate for A. However, the concentration of B is changing twice as fast as the concentration of A, so we need to multiply the rate from the previous step by 2 to get the average rate of change of B. I hope this helps!
. Since we only have data for the reactant, we should use the first version of the average rate equation. This gives us -(final concentration of A - initial concentration of A) / (final time - initial time). This will give us the average rate for A. However, the concentration of B is changing twice as fast as the concentration of A, so we need to multiply the rate from the previous step by 2 to get the average rate of change of B. I hope this helps!
-
Frankie Mele 3J
- Posts: 104
- Joined: Wed Sep 30, 2020 10:09 pm
Re: sapling #2
First, you can find the rate at which A is changing using the equation delta[A]/deltat and insert values from the chart provided. From the equation, we know that B is being produced at two times the decreasing rate of A (-rateA=1/2rateB --> rateB=-2*rateA). We can then use the value we found for the rate of A to solve for rateB.
-
Daniela Santana 2L
- Posts: 103
- Joined: Wed Sep 30, 2020 9:59 pm
Re: sapling #2
Hi! I solved this problem by taking the concentration of the second time given (t=292) and of the first time given (t=0) and subtracting them from each other and then dividing them by the overall change in time (292). I then multiplied the value I got by two since B has a coefficient of 2. This should be the answer, I hoped this helped :)
Return to “Method of Initial Rates (To Determine n and k)”
Who is online
Users browsing this forum: No registered users and 5 guests

