So my problem 12 states:
A particular reactant decomposes with a half‑life of 101 s when its initial concentration is 0.338 M. The same reactant decomposes with a half‑life of 241 s when its initial concentration is 0.142 M.
Determine the reaction order.
a.)1
b.)2
c.)3
I was wondering how you would find the order with just the given info from the problem.
Thank you in advance!
Sapling week 9-10. #12
Moderators: Chem_Mod, Chem_Admin
-
IanWheeler3F
- Posts: 100
- Joined: Wed Sep 30, 2020 9:49 pm
Re: Sapling week 9-10. #12
so the general equation for a half life for an order greater than one (n>1) is (k)(C_{o}^{n-1})}) so if you divide some half life t2 with initial concentration C2 over another half life t1 with initial concentration C1 you can derive the equation
so if you divide some half life t2 with initial concentration C2 over another half life t1 with initial concentration C1 you can derive the equation 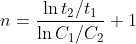 so in your case it becomes (ln(241/101))/(ln(0.338/0.142)) + 1 = 2.0028 which is about 2
so in your case it becomes (ln(241/101))/(ln(0.338/0.142)) + 1 = 2.0028 which is about 2
I don't know how else to do it i'm sure there's an easier way, i just think this equation is neat
I don't know how else to do it i'm sure there's an easier way, i just think this equation is neat
-
Andrew Wang 1C
- Posts: 101
- Joined: Wed Sep 30, 2020 10:11 pm
- Been upvoted: 5 times
Re: Sapling week 9-10. #12
Given that the half-life changes when concentration changes, you can deduce that it isn't a first-order reaction. And I don't think we covered 3rd order reactions in lecture, so that should leave you with 2nd order.
-
Anh Trinh 1J
- Posts: 156
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Sapling week 9-10. #12
In the first half-life, it is 101 s with initial concentration of 0.338 M, while in the second half-life it is 241 s with initial concentration of 0.142 M, and by comparing the four numbers, we can determine that the half-life increased when initial concentration decreased, so the half-life has an inverse relationship with initial concentration. From the zero-order, first-order, and second-order half-life equations, only the second-order half-life equation shows this inverse relationship, so the reaction order must be 2.
-
Anh Trinh 1J
- Posts: 156
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Sapling week 9-10. #12
In the first half-life, it is 101 s with initial concentration of 0.338 M, while in the second half-life it is 241 s with initial concentration of 0.142 M, and by comparing the four numbers, we can determine that the half-life increased when initial concentration decreased, so the half-life has an inverse relationship with initial concentration. From the zero-order, first-order, and second-order half-life equations, only the second-order half-life equation shows this inverse relationship, so the reaction order must be 2.
-
Saumya Tawakley 1E
- Posts: 104
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Sapling week 9-10. #12
I found that an easier way to do this problem was to just plug in the numbers we know to each half-life reaction equation for 1st, 2nd, and 3rd order reactions and calculate k. So essentially we would calculate k for the half life of 101s then calculate k again for the half life of 241s for each order. Whichever order's half life equations give the same k value is the order of the reaction.
Return to “Method of Initial Rates (To Determine n and k)”
Who is online
Users browsing this forum: No registered users and 2 guests

