Zero order integrated rate law
Moderators: Chem_Mod, Chem_Admin
-
torialmquist1F
- Posts: 49
- Joined: Sat Jul 22, 2017 3:00 am
Zero order integrated rate law
Can someone integrate the rate law for 0 order reactions step by step? Im having trouble visualizing how it gets to [A]o-[A]=kt
-
Yashaswi Dis 1K
- Posts: 56
- Joined: Fri Sep 29, 2017 7:04 am
Re: Zero order integrated rate law
Sure. So here's how it goes:
Rate = -d[A]/dt = k[A]0 for zero order reactions where [A]0 = 1, so rate = -d[A]/dt = k.
Then you have to do a method called separation of variables which is used in calculus where you multiply the dt to the other side to get all the d(stuff) on each side (kind of like multiplying by the denominator of a fraction on both sides like if it's 1/3=k, you are doing 1= 3k, except instead of using concrete numbers you are using the derivative d(stuff)).
Here's what you get when you do separation of variables:
-d[A] = k dt
Then you integrate both sides (notice I also multiplied the negative over so it's easier):
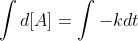
Then you get (because integral of dx is x and integral of a constant k is k times the variable t, where in this case x is [A]):
[A]t - [A]0= -kt
Notice that I made it into a definite integral from time t=0 to final time t so you don't really need a constant.
So then, I solve for [A]t by moving over the [A]0 over to the right:
[A]t = -kt + [A]0.
Can also check out this video for more details on the process I described: https://www.youtube.com/watch?v=C2LK0CWmmq4
Hopefully that gives a clearer idea!
Rate = -d[A]/dt = k[A]0 for zero order reactions where [A]0 = 1, so rate = -d[A]/dt = k.
Then you have to do a method called separation of variables which is used in calculus where you multiply the dt to the other side to get all the d(stuff) on each side (kind of like multiplying by the denominator of a fraction on both sides like if it's 1/3=k, you are doing 1= 3k, except instead of using concrete numbers you are using the derivative d(stuff)).
Here's what you get when you do separation of variables:
-d[A] = k dt
Then you integrate both sides (notice I also multiplied the negative over so it's easier):
Then you get (because integral of dx is x and integral of a constant k is k times the variable t, where in this case x is [A]):
[A]t - [A]0= -kt
Notice that I made it into a definite integral from time t=0 to final time t so you don't really need a constant.
So then, I solve for [A]t by moving over the [A]0 over to the right:
[A]t = -kt + [A]0.
Can also check out this video for more details on the process I described: https://www.youtube.com/watch?v=C2LK0CWmmq4
Hopefully that gives a clearer idea!
Return to “Zero Order Reactions”
Who is online
Users browsing this forum: No registered users and 4 guests

