zero order rate?
Moderators: Chem_Mod, Chem_Admin
-
Jasmine Botello 2F
- Posts: 67
- Joined: Fri Sep 29, 2017 7:04 am
zero order rate?
In my notes, I wrote down that for a zero order the rate does not depend on concentration.. is this correct or did I write down incorrect information??
-
Jessica Lutz 2E
- Posts: 56
- Joined: Fri Sep 29, 2017 7:04 am
Re: zero order rate?
This is true! You can see from the equation [A]=-kt+[A]o that the concentration of A changes only as a result of k and time, not because of its own concentration like in the first order equation (that uses ln[A]) or the second order equation (that uses 1/[A]).
-
Jasmine Botello 2F
- Posts: 67
- Joined: Fri Sep 29, 2017 7:04 am
-
Nina Gautam 1K
- Posts: 52
- Joined: Fri Sep 29, 2017 7:04 am
Re: zero order rate?
It's not necessarily because the first and second order equations start with ln[A] and 1/[A] instead of just [A], those are just mathematical functions that make it easier to understand the other side of the equation. The rate of reaction in first and second reactions depend on the concentration on initial reaction. However, zero order reactions occur at a rate that is constant throughout the reaction. This can occur if there is a catalyst or enzyme present, for example, which controls the speed of the reaction no matter how much reactant is present.
-
William Satyadi 2A
- Posts: 31
- Joined: Sat Jul 22, 2017 3:00 am
Re: zero order rate?
That is correct; a zero order reaction rate does not depend on the concentration of the reactant present, as long as there is some reactant. When integrating the rate law, we get Rate = 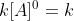 . Since anything raised to the zero power is 1, we can see that the rate just depends on k.
. Since anything raised to the zero power is 1, we can see that the rate just depends on k.
-
Jennie Fox 1D
- Posts: 66
- Joined: Sat Jul 22, 2017 3:01 am
Re: zero order rate?
True, in zero order reactions the rate does not depend on concentration. d[A]=-kdt
-
Shreya Ramineni 2L
- Posts: 50
- Joined: Fri Sep 29, 2017 7:07 am
Re: zero order rate?
This is true; rate is independent of concentration, as the differential rate law is rate=k ([A]^0=1).
-
Caroline LaPlaca
- Posts: 30
- Joined: Sat Jul 22, 2017 3:01 am
-
snehabhargava
- Posts: 52
- Joined: Thu Jul 13, 2017 3:00 am
-
Pooja Nair 1C
- Posts: 55
- Joined: Thu Jul 13, 2017 3:00 am
Re: zero order rate?
Yup! Zero order rates are independent of concentration, but assumes that there is some reactant.
-
vaishali 1D
- Posts: 61
- Joined: Fri Sep 28, 2018 12:19 am
Re: zero order rate?
That's correct! zeroth-order reactions have rates that won't vary when the reactant concentrations change. If the concentrations of reactants change and the rate remains the same, the reaction is zero order.
-
Chloe Qiao 4C
- Posts: 65
- Joined: Fri Sep 28, 2018 12:27 am
Re: zero order rate?
It is correct. Zero order reaction usually involves a catalyst or enzyme that works at its maximum potential. Increasing concentration cannot increase the rate the catalyst or enzyme works, and therefore zero order reactions do not depend on the concentration.
Re: zero order rate?
This is true. For a zero order, Rate = K(conc) raised to power 0. So it is just K.
-
Calvin Patel 2H
- Posts: 37
- Joined: Fri Sep 28, 2018 12:22 am
Re: zero order rate?
You wrote down the correct information. Zero order reactions are not dependent on the concentrations of the reactant
Return to “Zero Order Reactions”
Who is online
Users browsing this forum: No registered users and 2 guests

