Half life
Moderators: Chem_Mod, Chem_Admin
-
Abigail Menchaca_1H
- Posts: 104
- Joined: Sat Sep 07, 2019 12:19 am
-
Adam Kramer 1A
- Posts: 103
- Joined: Sat Aug 24, 2019 12:15 am
Re: Half life
to find the half life of the zero order reaction, you need to use the equation [A.5]=-kt+[A0], where A.5 is half the concentration of A0
+
+
-
Serena Song 1A
- Posts: 100
- Joined: Wed Sep 30, 2020 9:53 pm
- Been upvoted: 1 time
-
clairehathaway 2J
- Posts: 112
- Joined: Wed Sep 30, 2020 9:38 pm
Re: Half life
Today in lecture (3/3) Dr. Lavelle went through the derivation of finding the equation for the half-life of a zero-order reaction.
He started with the equation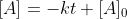 , then when looking for
, then when looking for 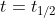 we know that
we know that 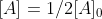 . Then we can find that
. Then we can find that
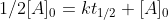 which simplifies to
which simplifies to 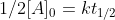 and ultimately gives us the zero-order half life equation which is:
and ultimately gives us the zero-order half life equation which is: 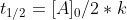
He started with the equation
-
Xinying Wang_3C
- Posts: 105
- Joined: Wed Sep 30, 2020 9:39 pm
-
Samantha Lee 1A
- Posts: 108
- Joined: Wed Sep 30, 2020 10:05 pm
- Been upvoted: 1 time
Re: Half life
The half life of a zero order reaction is 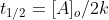
The derivation of this equation was in 3/3 lecture. The integrated rate law for the zero order is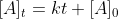 . At
. At 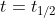 , [A] = 1/2*([A]_0). When we substitute 1/2[A]_0 into the equation for [A], the equation can be rearranged. The initial concentrations of A can be combined and then we solve for
, [A] = 1/2*([A]_0). When we substitute 1/2[A]_0 into the equation for [A], the equation can be rearranged. The initial concentrations of A can be combined and then we solve for 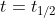 . When we solve for
. When we solve for 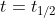 , we get the half life equation for the zero order.
, we get the half life equation for the zero order.
We can either derive the equation every time you want to use the half life equation for each order, using its specific integrated rate law, or you can use the answer of the derivation. The half life equation will be the same for every problem that is of that order.
The derivation of this equation was in 3/3 lecture. The integrated rate law for the zero order is
We can either derive the equation every time you want to use the half life equation for each order, using its specific integrated rate law, or you can use the answer of the derivation. The half life equation will be the same for every problem that is of that order.
-
Stephen Min 1I
- Posts: 100
- Joined: Wed Sep 30, 2020 10:01 pm
-
rhettfarmer-3H
- Posts: 122
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Half life
To begin, Half life is when the reaction reaches half the initial amount. Hence, 0.5A=-kt+A. Then becomes -.5A=-kt which more so .5A=kt. So then we get A*1/(2*k)=t. That the half life of zero reaction.
-
Brian_Wu_3B
- Posts: 103
- Joined: Wed Sep 30, 2020 9:41 pm
-
abby hyman
- Posts: 131
- Joined: Thu Jul 25, 2019 12:16 am
Re: Half life
You must use the specific half life equation for a zero order reaction which is t1/2 = [A]0/2k
-
aashmi_agrawal_3d
- Posts: 116
- Joined: Wed Sep 30, 2020 9:39 pm
-
David Liu 1E
- Posts: 100
- Joined: Wed Sep 30, 2020 10:07 pm
Re: Half life
we use the equation specifically for half life for zero order reactions! which is the equation everyone else has stated
-
Joshua Swift
- Posts: 100
- Joined: Wed Sep 30, 2020 9:50 pm
-
Edgar Velazquez 2K
- Posts: 50
- Joined: Wed Nov 20, 2019 12:20 am
Re: Half life
I believe you can use the half-life equation where you divide [A]/2k. The first step is to find the original concentration of the given molecule.
-
Neha Mukund
- Posts: 109
- Joined: Fri Sep 24, 2021 5:23 am
Re: Half life
In order to determine the half-life of a reaction, you can begin by using this equation:
t1/2 = [A]0/2k
t1/2 = [A]0/2k
Return to “Zero Order Reactions”
Who is online
Users browsing this forum: No registered users and 4 guests

