Sapling HW Week 9/10 #4
Moderators: Chem_Mod, Chem_Admin
-
Adam_Ventura_1H
- Posts: 108
- Joined: Wed Sep 30, 2020 10:05 pm
Sapling HW Week 9/10 #4
I am having trouble conceptually understanding how to obtain the proper units of the rate constant,k, for the different reaction orders (Zeroth Order, First Order, Second Order, and Third Order). Is there a way to figure this out or is it something that we are just supposed to know?
-
Megan Chan 3A
- Posts: 104
- Joined: Wed Sep 30, 2020 9:50 pm
Re: Sapling HW Week 9/10 #4
The unit for rate is molarity per second (M/s) so depending on the order of the reaction, you will determine the units for k that will cancel the other terms to give you the units for rate.
For example, if you have a rate law: Rate = k[A]^2[B] the overall order of the reaction is 3, so you have M^3 as the units in the numerator. In order to get M/s for rate, your rate constant must have the units M^-2.s^-1
Hopefully this helps!
For example, if you have a rate law: Rate = k[A]^2[B] the overall order of the reaction is 3, so you have M^3 as the units in the numerator. In order to get M/s for rate, your rate constant must have the units M^-2.s^-1
Hopefully this helps!
-
Josh Chou 3K
- Posts: 108
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 3 times
Re: Sapling HW Week 9/10 #4
You can figure it out because the reaction rate should be in M/s. If the equation for a reaction rate is rate = k [A]a, [A] represents the concentration of the reactant in M and "a" represents the order (and stoichiometric coefficient). A zero-order reaction would have the equation rate = k[A]0, so k would need to have the units M/s in order for the reaction rate to be in M/s. Similarly, a first-order reaction would have the equation rate = k[A]1, so k would need to have the units 1/s for the reaction rate to have the units M/s. A second-order reaction would have the equation rate = k[A]2, and because [A]2 would have units M2, k would need to have the units 1 / (M s) in order for the reaction rate to be in M/s. Essentially, the units of k will adjust so that the reaction rate comes out in M/s, and you can solve for the units of k, so you shouldn't have to worry about memorizing anything
-
Joshua Eidam 2A
- Posts: 89
- Joined: Wed Sep 30, 2020 9:58 pm
Re: Sapling HW Week 9/10 #4
You need to look at how the elements that make up the rate constant. For example, when trying to find the units for the rate constant of the 3rd Order Reaction, look at the units of the rate(M/s) and the units of the concentration term(M3).The unit of the rate constant is obtained by dividing the unit of the rate by the unit of the concentration term. Therefore, the rate constant would be 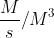 or
or 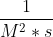
-
Kamille Kibria 2A
- Posts: 101
- Joined: Wed Sep 30, 2020 9:52 pm
Re: Sapling HW Week 9/10 #4
this question asks you to solve for k and give the units: rate has units of M/s and the concentration of any given molecule has units of M
- for the 0 order one, rate = k so M/s = k
- for the 1st order rate = kM so M/s = kM so the answer is 1/s when you divide by M
- same method for the 2nd and 3rd order rxns
- for the 0 order one, rate = k so M/s = k
- for the 1st order rate = kM so M/s = kM so the answer is 1/s when you divide by M
- same method for the 2nd and 3rd order rxns
Return to “Zero Order Reactions”
Who is online
Users browsing this forum: No registered users and 3 guests

