1. A reactant is in the FAST elementary step, so since this step is so fast, increasing the reactant does not make it faster.
2. When the effect of catalysts or enzymes are saturated, meaning that increasing the concentration of a reactant does not speed up reaction. (so the rate constant k is already so large (cause of catalyst) that increased reactant does nothing relatively)
I read in the website https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/02%3A_Reaction_Rates/2.10%3A_Zero-Order_Reactions
that zero order happens under these conditions: (such a good website called chem.libretexts)
1. Only a small fraction of the reactant molecules are in a location or state in which they are able to react, and this fraction is continually replenished from the larger pool. (which is the case especially when a rxn is catalyzed by attachment to a solid surface (heterogeneous catalysis) or to an enzyme)
- which I think Khan academy explains well why this is the case: https://www.khanacademy.org/science/high-school-biology/hs-energy-and-transport/hs-enzymes/a/hs-enzymes-review
2. When two or more reactants are involved, the concentrations of some are much greater than those of others
I don't understand well number two, but number 1 is consistent with Dr. Lavelle's number 2!!! Also, based on Khan academy's explanation, I want to add that rule # 1 also applies to catalyst concentration, because when there's too much catalyst, all substrates are bound already. We know that catalyst increases rxn rate, but it will not increase the rate under above conditions.
So maybe we can say that it is zero order for the catalyst although I don't think this is the official way to state haha.
So my question is: disregarding the FASt elementary step condition, in a rxn which can be catalyzed, does removing or adding a catalyst (or enzyme) change the order of a "zero-order reactant"?
________________________________________________________________________________________________________________________________________________
My own answer is... yes! Because imagine a condition where there are unlimited catalysts (enzyme), then there would no saturated substrate level because their always a hole in the enzyme to plug in a substrate. Basically # of enzyme active site = INFINITY ~ and beyond.
What's more interesting is that suppose you remove the catalyst completely, then there are no limited numbers of catalyst(enzyme) and substrate interaction because there's nothing at all!! So in this case, is the original "zero-order reactant" still 0 order?? I think not! I think this will shift the rate law and the zero-order reactant would have a different order.
I have evidence to support this claim.
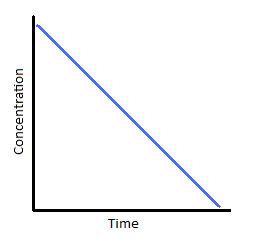
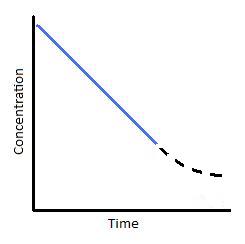
Figure 2: (left) Concentration vs. time of a zero-order reaction. (Right) Concentration vs. time of a zero-order catalyzed reaction.
pictures are from chem.libretexts
As stated from the site: "As a reaction progresses through time, however, it is possible that less and less substrate will bind to the catalyst. As this occurs, the reaction slows and we see a tailing off of the graph (Figure 2; right)."
Sooooo..... from this now I know that 0-order is "condition-dependent". It exists only under certain circumstances. The point that the rate changes(dotted lines) is the point when some catalysts have unbound active sites! So as substrate concentration decreases, more and more catalysts or active sites (active site is only for enzymes) are unused, compared to the saturated condition when all catalysts are used even when substrate concentration increases or decreases. BUT, when the substrate amount is lower than the catalyst amount, this dotted line curve APPEARS! WOW~
Ok. back to my point. If there are no catalysts at all, there is no problem with saturation at all, and thus... the "zero-order reactant" would not be zero order, as is also the case in website's example.
Lastly, to sum up, I want to quote the awesome chemistry free textbook web chem.libretexts: "Zero-order kinetics is always an artifact of the conditions under which the reaction is carried out. For this reason, reactions that follow zero-order kinetics are often referred to as pseudo-zero-order reactions. Clearly, a zero-order process cannot continue after a reactant has been exhausted. Just before this point is reached, the reaction will revert to another rate law instead of falling directly to zero"
Thank you for reading and I hope someone can provide some interesting thoughts.

