Concept of Half Life [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Vera Ong 3H
- Posts: 26
- Joined: Fri Jun 17, 2016 11:28 am
Concept of Half Life
Conceptually, how would you explain why a first order half life does not depend on the initial concentration?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Concept of Half Life [ENDORSED]
The half-life for a first-order reaction can be given as follows:
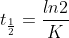
The natural log of 2 is a constant, so the half life only depends on the rate constant k. The rate constant is a function of temperature as opposed to concentration. Therefore, the half life is independent of concentration and only dependent on temperature.
The natural log of 2 is a constant, so the half life only depends on the rate constant k. The rate constant is a function of temperature as opposed to concentration. Therefore, the half life is independent of concentration and only dependent on temperature.
-
Michael Lesgart 1H
- Posts: 26
- Joined: Wed Sep 21, 2016 2:57 pm
Re: Concept of Half Life
Is there a general trend that relates temperature and half-life? Does a higher temperature correlate to a lower half live or vice versa?
-
Vera Ong 3H
- Posts: 26
- Joined: Fri Jun 17, 2016 11:28 am
Re: Concept of Half Life
i believe there would be a trend since the half life of a first order reaction depends on the reaction constant. We will go over the arrhenius equation later on which will show how activation energy, temperature, and collision frequency all affect the rate constant, thus affecting the half life. and to answer your question, i think a higher temperature leads to a lower half life
Return to “First Order Reactions”
Who is online
Users browsing this forum: No registered users and 2 guests

