HW #14.35b
Moderators: Chem_Mod, Chem_Admin
-
Shannon Han 2B
- Posts: 48
- Joined: Fri Sep 26, 2014 2:02 pm
HW #14.35b
I'm confused on 35b, where the question asks to find the time it takes for the concentration of SO2Cl2 to decrease to 10% of its initial concentration. Why does the solutions manual just do ln10, and when I solved ln10/k like they did, I got 0.000819 minutes, not 819 minutes. Could someone help explain this?
-
Neil DSilva 1L
- Posts: 70
- Joined: Fri Sep 26, 2014 2:02 pm
Re: HW #14.35b
The ln10 comes from a modified version of the first-order half-life equation. Instead of using ln2 (which equals 0.693, the number we see in the half-life equation) which comes from the following analysis:
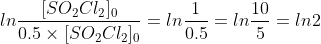 ,
,
the solutions manual is finding the time it takes the concentration to decrease to 10% (aka finding the "tenth-life") using the following steps:
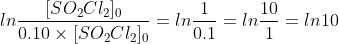 .
.
Hopefully that helps you understand that portion. As for your calculations, you might have inputted the numbers incorrectly. When I do the calculations, I get and when you divide that by k (
and when you divide that by k ( ), you should get 819 minutes. How did you input your data?
), you should get 819 minutes. How did you input your data?
the solutions manual is finding the time it takes the concentration to decrease to 10% (aka finding the "tenth-life") using the following steps:
Hopefully that helps you understand that portion. As for your calculations, you might have inputted the numbers incorrectly. When I do the calculations, I get
-
Sonia Kumar 2A
- Posts: 6
- Joined: Fri Sep 26, 2014 2:02 pm
Re: HW #14.35b
If you were also confused on the ln 10, I was originally confused on why it was ln 10 and not ln(.1) and then I noticed that by dividing the negative it flipped the fraction.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: HW #14.35b
Neil is correct. From the 1st order integrated rate law, if we want to find the time to drop from [A] to [A]/n, eg. the "n-th life"
ln([A]/n) = -kt + ln[A]
ln(1/n) = -kt
ln(n) / k = t
Then the n-th life is just ln(n)/k. So the half life is ln2/k, the tenth life is ln10/k, etc.
ln([A]/n) = -kt + ln[A]
ln(1/n) = -kt
ln(n) / k = t
Then the n-th life is just ln(n)/k. So the half life is ln2/k, the tenth life is ln10/k, etc.
Return to “First Order Reactions”
Who is online
Users browsing this forum: No registered users and 5 guests

