1st Order Reactions
Moderators: Chem_Mod, Chem_Admin
-
Rylee Mangan 1K
- Posts: 102
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 2 times
1st Order Reactions
I am little confused. In monday's lecture I thought Dr. Lavelle said a straight decreasing slope indicated a first order reaction reaction today's lecture he said a decreasing exponential graph was a first order reaction. Can anyone clarify this?
-
Praneetha Kakarla 3A
- Posts: 104
- Joined: Wed Sep 30, 2020 9:33 pm
- Been upvoted: 2 times
Re: 1st Order Reactions
Hey, yor a 1st order rxn, plotting ln[A] over time gives a straight line, while plotting [A] over time gives a decreasing exponential graph. The y-axis is different.
-
Lindsey_Su_3A
- Posts: 113
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 2 times
Re: 1st Order Reactions
If the graph of [A] vs. time is decreasing exponentially, it is first order.
If the graph of ln[A] vs. time is decreasing linearly, it is first order.
The difference is the Y axis
If the graph of ln[A] vs. time is decreasing linearly, it is first order.
The difference is the Y axis
-
Kiyoka Kim 3C
- Posts: 108
- Joined: Wed Sep 30, 2020 9:59 pm
Re: 1st Order Reactions
I believe you get different graphs depending on what your y-axis is. When the y-axis is ln[reactant] you get a linear graph and when the y-axis is [reactant] you get an exponential graph for first order reactions.
-
Stephen Min 1I
- Posts: 100
- Joined: Wed Sep 30, 2020 10:01 pm
Re: 1st Order Reactions
A first order reaction plot would be a decreasing linear slope when it is defined by ln[A] vs time, whereas it would be exponential when it is defined by [A] vs time (which would usually be the parameters for a 0 order reaction).
-
Ephrem Gerald 2A
- Posts: 56
- Joined: Wed Nov 18, 2020 12:26 am
Re: 1st Order Reactions
You can tell if a reaction is first order if the graph of ln[A] vs time is a negative linear graph. So if you see a negative slope line and the y-axis has the ln[A] plotted, you know that the reaction must be first order.
-
KhanTran3K
- Posts: 100
- Joined: Wed Sep 30, 2020 9:31 pm
Re: 1st Order Reactions
When we plot a 1st order reaction, the y axis is actually the ln[A] rather than just A. The ln[A] give us this linear relationship while just [A] vs time gives us a decreasing exponential function. With this, they both still show us a 1st order reaction. I hope this helps!
-
Annie Liang 3D
- Posts: 50
- Joined: Mon Jun 17, 2019 7:25 am
Re: 1st Order Reactions
We can tell if the reaction is a first order reaction if the graph for ln[A] vs. time is linear with a slope of -k.
-
Anh Trinh 1J
- Posts: 156
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: 1st Order Reactions
For a first order reaction, you would plot ln[A] vs time, with ln[A] as the y axis and time as the x axis. You are plotting the equation 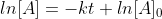 , and this equation has a similar form to the equation
, and this equation has a similar form to the equation  . Y in this equation is ln[A], m (the slope) is -k, x is time, and C (y-intercept) is
. Y in this equation is ln[A], m (the slope) is -k, x is time, and C (y-intercept) is 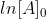 . If it is a straight line, then the slope is -k, and it is a first order reaction.
. If it is a straight line, then the slope is -k, and it is a first order reaction.
If you plot [A] vs time, it would be an exponential decay plot. When the concentration of reactant ([A]) is decreasing exponentially, it means it is a first order reaction. Then, when you take the natural log, you will get a linear plot, which is ln[A] vs time.
If you plot [A] vs time, it would be an exponential decay plot. When the concentration of reactant ([A]) is decreasing exponentially, it means it is a first order reaction. Then, when you take the natural log, you will get a linear plot, which is ln[A] vs time.
-
Gigi Elizarraras 2C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:41 pm
Re: 1st Order Reactions
The ln[a] graph will be negatively linear whereas the [a] graph will be exponentially decreasing. Both graphs represent the first order, but it is only the y-axis value that is different
Hope this helps:)
Hope this helps:)
-
Samudrala_Vaishnavi 3A
- Posts: 103
- Joined: Wed Sep 30, 2020 9:34 pm
Re: 1st Order Reactions
So the straight line is essentially an exponential graph (differential rate law), but the natural log (integrated rate law) is taken of both sides to make it a clearly inversely proportional straight line that is easier to analyze.
-
Jason Knight - 1F
- Posts: 113
- Joined: Wed Sep 30, 2020 9:55 pm
Re: 1st Order Reactions
The straight-line graph for first order is when you plot ln[A] versus time, and the plot [A] versus time is the decreasing exponential graph.
Re: 1st Order Reactions
https://www.chem.purdue.edu/gchelp/howtosolveit/Kinetics/IntegratedRateLaws.html
This was a good visualization to help me under understand rate laws, hope it helps!
This was a good visualization to help me under understand rate laws, hope it helps!
-
MariaCassol1L
- Posts: 49
- Joined: Wed Nov 13, 2019 12:18 am
Re: 1st Order Reactions
So you are simply graphing different equations. So ln[A]= -kt+[A]0 (ln[A] gives a straight line), you can modify that to get [A]=[A]0e^-kt and if you plot that you get a decreasing exponential.
-
Tanner Bartyczak 1K
- Posts: 100
- Joined: Wed Sep 30, 2020 9:51 pm
Re: 1st Order Reactions
A straight line represents the order based on one of the three formulas. A first order reaction is a straight line when graphing ln[A], a second order reaction is a straight line when graphing 1/[A], and a zero order reaction is a straight line when graphing [A].
Re: 1st Order Reactions
Zero order: [A] gives a straight line
First order: ln([A]) gives a straight line
Second order: 1/[A] gives a straight line
First order: ln([A]) gives a straight line
Second order: 1/[A] gives a straight line
-
Rob Tsai 2F
- Posts: 106
- Joined: Wed Sep 30, 2020 10:09 pm
Re: 1st Order Reactions
Dr. Lavelle was simply using a different graph when he was describing the rates of first order reactions. In the first graph, a negative linear result in the graph of ln[A] vs. time would be indicative of a first order reaction. in the second graph, a negative exponential result in the graph of [A] vs time would be indicative of a first order reaction. It is simply the difference in y-axis that causes the difference in the graphs. Remember, Dr. Lavelle noted that we do this because it is always easier to make observations of something linear rather than something that is exponential, which is why we take the natural log of [A]. Hope this helps!
-
Eric Cruz 2G
- Posts: 97
- Joined: Wed Sep 30, 2020 9:45 pm
- Been upvoted: 1 time
Re: 1st Order Reactions
A straight decreasing slope for first order occurs when the y axis relates to ln[A]. However, if you graph [A] over time and its 1st order, it would have an exponential decrease.
-
Kristina Krivenko 3I
- Posts: 118
- Joined: Wed Sep 30, 2020 9:52 pm
- Been upvoted: 1 time
Re: 1st Order Reactions
Hi,
So both graphs would indicate that it is a first order reaction. However, the difference is what you plot on the axes.
If you plot concentration of the reactant (y-axis) vs time (x-axis) and get a decreasing exponential graph, it is a first order reaction.
If you plot natural log of the concentration of the reactant (y-axis) vs time (x-axis) and get a straight line with a negative slope, it is a first order reaction.
Hope this helps :)
So both graphs would indicate that it is a first order reaction. However, the difference is what you plot on the axes.
If you plot concentration of the reactant (y-axis) vs time (x-axis) and get a decreasing exponential graph, it is a first order reaction.
If you plot natural log of the concentration of the reactant (y-axis) vs time (x-axis) and get a straight line with a negative slope, it is a first order reaction.
Hope this helps :)
-
Nicoli Peiris 1B
- Posts: 110
- Joined: Wed Sep 30, 2020 10:02 pm
Re: 1st Order Reactions
For first order a straight line should be shown for ln[x] versus time and exponentially decreasing for [x] versus time.
-
Ria Nawathe 1C
- Posts: 108
- Joined: Wed Sep 30, 2020 9:39 pm
- Been upvoted: 3 times
Re: 1st Order Reactions
Both graphs describe 1st order reactions, the difference is that one expresses the integrated rate law while the other is based on the differential rate law.
-
Daniel Huynh 2J
- Posts: 51
- Joined: Wed Jan 08, 2020 12:16 am
Re: 1st Order Reactions
When you graph ln[A] vs. Time and the points approximate a straight line with downward slope, then you can infer that it is a first order reaction.
-
Kylie Joe 2A
- Posts: 52
- Joined: Wed Nov 18, 2020 12:22 am
Re: 1st Order Reactions
This is dependent on the axes of the graph you are considering. A linear graph can indicate (if I remember correct) every order, depending on what two variables you are comparing
-
Brian Acevedo 2E
- Posts: 69
- Joined: Wed Sep 30, 2020 9:44 pm
Re: 1st Order Reactions
With regards to first order reactions, you would see a straight line with a slope of -k when you plot the natural log of the concentration in question (on the vertical axis) against time (horizontal axis). If you were to plot the concentration of that same reactant without taking the natural log against time, you would get a graph that indicates exponential decay. Both are possible results when a reactant is first order, just be sure to interpret the graph accordingly given what you chose for the vertical axis.
-
Neha Jonnalagadda 2D
- Posts: 110
- Joined: Fri Sep 24, 2021 7:06 am
-
Jacob_Eberson_2D
- Posts: 50
- Joined: Mon Jan 03, 2022 8:40 pm
Re: 1st Order Reactions
when the y-axis is ln[A] it's linear for the 1st order, and when it's [A] vs time the graph is exponential
-
almaortega
- Posts: 104
- Joined: Fri Sep 24, 2021 6:54 am
Re: 1st Order Reactions
For first order reactions, plotting [A] vs time will produce a decreasing exponential graph, and ln[A] vs time will produce a decreasing linear graph which makes finding slope or k easier. It just depends on the derivation you do.
-
Kelly McFarlane
- Posts: 101
- Joined: Fri Sep 24, 2021 6:02 am
Re: 1st Order Reactions
In a first order reaction, it depends on the axis as to whether the plot is linear or exponential. If the y-axis is ln[A], then the graph is linear with a negative slope while if the y-axis is just [A], then the plot is decreasing exponential.
Return to “First Order Reactions”
Who is online
Users browsing this forum: No registered users and 2 guests

