Order of Reactions
Moderators: Chem_Mod, Chem_Admin
-
Rachel Kwan 1B
- Posts: 58
- Joined: Wed Nov 18, 2020 12:23 am
Order of Reactions
Are the orders of reactions based on the number of reactants in an equation? Can someone clarify what each order means and how we can recognize them in equations?
-
Stuti Pradhan 2J
- Posts: 173
- Joined: Wed Sep 30, 2020 9:32 pm
- Been upvoted: 5 times
Re: Order of Reactions
The order of the reaction is not based on the number of reactants in the equation but on the order of each individual reactant. You can add the exponents on all of the reactants to figure out the order of the reaction. The order of the reaction basically tells you how the rate constant is affected by the products. For example, for a zero-order reaction, the rate constant is not affected by a change in concentration of the reactant. For a first order reaction, the rate constant is directly proportional to the concentration of reactants, so doubling the concentration of reactants would double the rate. For a second-order reaction, the rate constant is proportional to the concentration of reactants squared, so doubling the concentration of reactants would quadruple the rate.
Hope this helps!
Hope this helps!
-
Manseej Khatri 2B
- Posts: 100
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Order of Reactions
The total order of a reaction is the sum of the orders of each reactant. Since its possible that not all reactants contribute to the rate of the reaction, the order is not necessarily based on the number of reactants in the equation.
To find the order you have to compare experiments. To find the order of one reactant, keep the other reactant the same concentration, and change the concentration of the reactant being analyzed. Then divide the two reactions Rate(exp 1) =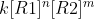 and Rate(exp2) =
and Rate(exp2) =  . K and one of the reactant expressions cancel since you kept that reactant concentration the same. Solve for the order (m or n) and that will be the order of the reactant. Repeat for the other concentration, add the two orders, and that will be the overall order.
. K and one of the reactant expressions cancel since you kept that reactant concentration the same. Solve for the order (m or n) and that will be the order of the reactant. Repeat for the other concentration, add the two orders, and that will be the overall order.
To find the order you have to compare experiments. To find the order of one reactant, keep the other reactant the same concentration, and change the concentration of the reactant being analyzed. Then divide the two reactions Rate(exp 1) =
-
Elizabeth Kaplan 3I
- Posts: 125
- Joined: Wed Sep 30, 2020 9:32 pm
Re: Order of Reactions
The orders of a reaction (also called the overall order) is calculated by adding up the orders of each reactant. To determine the order of each reactant, you either use the psuedo rate law (in which there is an excess in concentration of all but 1 reactant) or you compare experiments when all but one reactant are unchanging in their concentrations.
-
Nathan Lao 2I
- Posts: 100
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 1 time
Re: Order of Reactions
The short answer to your question is no. The long answer is that the order of a reaction is dependent on the orders of the reactants. The order of a reactant indicates how much the reactant affects the rate of the reaction. You would need to add up the orders of each reactant to get the overall order of the reaction, and finding the order of individual reactants really depends on the information given in the problem. This could be done by comparing reactant concentrations to instantaneous rates or looking at the reaction mechanism.
-
JoshMoore2B
- Posts: 100
- Joined: Wed Sep 30, 2020 9:51 pm
Re: Order of Reactions
Rachel Kwan 1B wrote:Are the orders of reactions based on the number of reactants in an equation? Can someone clarify what each order means and how we can recognize them in equations?
The orders of a reaction depends on the order of the reactants in the equation. If you have A + B -> C, and A is order 1 and B order 2, then the overall reaction is order 3. To find the orders of individual reactants, you have to compare experimental data.
-
Lung Sheng Liang 3J
- Posts: 100
- Joined: Wed Sep 30, 2020 9:33 pm
-
SavannahScriven_1F
- Posts: 107
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Order of Reactions
The only time the order can be determined for a reaction using stoichiometry is if the reaction is written as an elementary step. The overall reaction order cant be found using direct stoichiometry.
-
Shana Patel 1C
- Posts: 111
- Joined: Wed Sep 30, 2020 9:36 pm
Re: Order of Reactions
To get the overall order of the reactions, you need to add the orders together.
-
Susan Chamling 1F
- Posts: 101
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Order of Reactions
To get the reaction’s overall order, we just take the sum of the individual orders.
-
Neha Jonnalagadda 2D
- Posts: 110
- Joined: Fri Sep 24, 2021 7:06 am
-
14b_student 2E
- Posts: 118
- Joined: Fri Sep 24, 2021 7:18 am
Return to “First Order Reactions”
Who is online
Users browsing this forum: No registered users and 3 guests

