Determining the time needed for a concentration to fall
Moderators: Chem_Mod, Chem_Admin
-
Catherine Bubser 2C
- Posts: 114
- Joined: Wed Sep 30, 2020 9:45 pm
Determining the time needed for a concentration to fall
What values do we plug into the integrated rate laws for initial concentration and final concentration if we aren't given a concertation to start with? For example, if we were asked to calculate the time it took for something to fall to 10% of its initial concentration, how would we use this in the equation?
-
Peter DePaul 1E
- Posts: 101
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Determining the time needed for a concentration to fall
For the integrated Rate Laws, I can't really answer your question because it's kind of broad.
For the concentrations I would look to how we derived the formula for half life to calculate this time. I attached an image below (for first order reactions), and my idea is instead of saying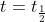 use
use  , and instead of having
, and instead of having 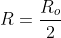 use
use 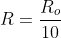 . I believe that should work out mathematically.
. I believe that should work out mathematically.
For the concentrations I would look to how we derived the formula for half life to calculate this time. I attached an image below (for first order reactions), and my idea is instead of saying
-
Catherine Bubser 2C
- Posts: 114
- Joined: Wed Sep 30, 2020 9:45 pm
-
Thomas Gimeno
- Posts: 95
- Joined: Wed Sep 30, 2020 9:49 pm
Re: Determining the time needed for a concentration to fall
if you want to test something like dropping 10% you can just use a hypothetical like plugging in your initial concentration as 1M and your final concentration as .9M. I'm not 100% sure cause I know the rate of change can be dependent on the morality for first order reactions so you mihgt have to know the initial concentration.
Return to “First Order Reactions”
Who is online
Users browsing this forum: No registered users and 3 guests

