Page 1 of 1
3rd order?
Posted: Tue Feb 16, 2016 8:13 pm
by Nadege Diedrich 1H
What is the units for third order rxns?
Re: 3rd order?
Posted: Tue Feb 16, 2016 8:13 pm
by Betty Tesfaye 2G
1/M^2s
Re: 3rd order?
Posted: Sat Mar 10, 2018 12:38 am
by Michael Lee 2I
I agree
Re: 3rd order?
Posted: Sat Mar 10, 2018 2:46 pm
by 204918982
If you don't remember the units for k for certain orders you can always just divide the rate by the concentration. For 3rd order reactions, the units are (M/s)(1/M^3) which simplifies to (1/M^2)s
Re: 3rd order?
Posted: Sat Mar 10, 2018 8:16 pm
by Cam Bear 2F
What would we be asked about third order reactions other than their units?
Re: 3rd order?
Posted: Sat Mar 10, 2018 11:15 pm
by Fatima_Iqbal_2E
If you want to write the units of K in terms of molarity, it would be
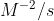
, but if you wanted to write it in terms of moles and liters, it would be
)
.
Re: 3rd order?
Posted: Fri Mar 16, 2018 2:46 am
by Pooja Nair 1C
If you look at first order and second order reactions, when you increase the order, you raise the power of the L and the mol in the units. Thus as you increase the order further, the power continues to rise.
Re: 3rd order?
Posted: Fri Mar 16, 2018 7:07 pm
by Rachel Wang
It's very rare for 3rd order rxns to occur unless it's the overall rxn's order. The units are 1/M^2.s