Slow and Fast Steps [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Valeria Quintana 1G
- Posts: 11
- Joined: Wed Sep 21, 2016 2:55 pm
Slow and Fast Steps
I was wondering if any of you could please explain why the the order for which the slow/fast steps of a reaction matters. Why do we use one way to solve for a reaction that is fast in the first step and is slow in the second step, and another way for a reaction that is slow in the first step and is fast in the second step?
-
Justin_Yi_1J
- Posts: 27
- Joined: Fri Jul 22, 2016 3:00 am
- Been upvoted: 1 time
Re: Slow and Fast Steps [ENDORSED]
If the mechanism is a slow step followed by a fast step, then the rate law of the slow step is the overall rate law. However, we must go through a different processes if the initial elementary is not slow. That is because any elementary step after the initial step will have an intermediate. So a slow step that is not the initial step will have an intermediate involved and therefore the slow step's rate law will have an intermediate. This is a problem because the slow step's rate law will not match the experimentally determined overall rate law which doesn't have the intermediate species in its expression. Therefore, we must use the pre-equilibrium method to express the slow step's rate law without any intermediates so it will match what is experimentally determined.
For example:
The experimentally determined rate law is k[A]2. The equation is 2A -> B
A mechanism for the reaction is
A -> C (fast) RATE = k1[A]
A + C -> B (slow) RATE = k2[A][C]
The slow step's rate law does not match the experimentally determined rate law because it contains [C], the intermediate. So we use the pre-equilibrium method to make it match.
A<->C, it bottlenecks b/c next step is so slow, so step 1 at "equilibrium." K = , [C] = K[A], substitute [C] in k2[A][C] for [C] = K[A]. So the rate law is
, [C] = K[A], substitute [C] in k2[A][C] for [C] = K[A]. So the rate law is
k2[A]K[A] = k2K[A]2 =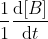 (rate of formation of B), so
(rate of formation of B), so
k2K[A]2 = k[A]2 where k = k2K.
The final rate law is k[A]2, which matches what we found in the experiment.
Hope this helps!
For example:
The experimentally determined rate law is k[A]2. The equation is 2A -> B
A mechanism for the reaction is
A -> C (fast) RATE = k1[A]
A + C -> B (slow) RATE = k2[A][C]
The slow step's rate law does not match the experimentally determined rate law because it contains [C], the intermediate. So we use the pre-equilibrium method to make it match.
A<->C, it bottlenecks b/c next step is so slow, so step 1 at "equilibrium." K =
k2[A]K[A] = k2K[A]2 =
k2K[A]2 = k[A]2 where k = k2K.
The final rate law is k[A]2, which matches what we found in the experiment.
Hope this helps!
-
Banik_Housepian_2K
- Posts: 39
- Joined: Wed Sep 21, 2016 2:59 pm
Return to “Second Order Reactions”
Who is online
Users browsing this forum: No registered users and 6 guests

