Finding out order
Moderators: Chem_Mod, Chem_Admin
-
ShravanPatel2B
- Posts: 100
- Joined: Fri Aug 30, 2019 12:18 am
Finding out order
Can the units of the rate constant in a rate law be used to identify the order of the reaction? Also would the units of the rate constant be different in in a rate law of species with different order?
-
Riya Sood 4G
- Posts: 160
- Joined: Sat Aug 24, 2019 12:18 am
Re: Finding out order
Yes the units of the rate constant can be used to find the order of the reaction.
Depending on the order, the units of the rate constant will be different. For example, for a first order reaction, the units of k will be s^-1.
The best way to ensure that you have the correct units is to plug in k and multiply the concentrations and it should also give the rate in terms of concentration/time
Depending on the order, the units of the rate constant will be different. For example, for a first order reaction, the units of k will be s^-1.
The best way to ensure that you have the correct units is to plug in k and multiply the concentrations and it should also give the rate in terms of concentration/time
-
Sebastian Lee 1L
- Posts: 157
- Joined: Fri Aug 09, 2019 12:15 am
- Been upvoted: 1 time
Re: Finding out order
Just to expand on the previous comment, you can generalize the rate constant's units as 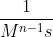 where n is the order of the reaction. So for a zero order reaction, the units would be M/s. For first order, it's just s^-1. For second order, it's
where n is the order of the reaction. So for a zero order reaction, the units would be M/s. For first order, it's just s^-1. For second order, it's 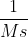 , etc.
, etc.
The rate constant's units will apply to the overall rate law of the step you are looking at. If you have a first order reactant and a second order reactant colliding, you can expect the units for a third order rate constant (assuming this is an elementary step).
The rate constant's units will apply to the overall rate law of the step you are looking at. If you have a first order reactant and a second order reactant colliding, you can expect the units for a third order rate constant (assuming this is an elementary step).
-
Jessa Maheras 4F
- Posts: 121
- Joined: Fri Aug 02, 2019 12:16 am
Re: Finding out order
Yes, the units of the rate constant are representative of the order of the reaction because the units of the rate constant must cancel out with tge units of the rate law.
-
Osvaldo SanchezF -1H
- Posts: 122
- Joined: Wed Sep 18, 2019 12:21 am
Re: Finding out order
The units do represent the rate constant but I would like to add that this is different to the total order of the whole reaction as a whole. You would add all of the constants to get the overall order. Do not forget to make that distinction.
-
William Chan 1D
- Posts: 102
- Joined: Sat Sep 14, 2019 12:15 am
Re: Finding out order
Technically, yes the rate order could be determined if you know the units of k. The units for k do change for different order equations. If you check to see that the units cancel out and are equal on both sides of the equation, you should be okay.
-
Bryan Chen 1H
- Posts: 58
- Joined: Mon Jun 17, 2019 7:24 am
-
Luc Zelissen 1K
- Posts: 57
- Joined: Mon Jun 17, 2019 7:23 am
Re: Finding out order
For order (m + n), the rate constant has units of mol1−(m+n)·L(m+n)−1·s−1
For order zero, the rate constant has units of mol·L−1·s−1 (or M·s−1)
For order one, the rate constant has units of s−1
For order two, the rate constant has units of L·mol−1·s−1 (or M−1·s−1)
And for order three, the rate constant has units of L2·mol−2·s−1 (or M−2·s−1)
For order zero, the rate constant has units of mol·L−1·s−1 (or M·s−1)
For order one, the rate constant has units of s−1
For order two, the rate constant has units of L·mol−1·s−1 (or M−1·s−1)
And for order three, the rate constant has units of L2·mol−2·s−1 (or M−2·s−1)
Re: Finding out order
Yes, the units can be used to determine the order of the reaction. The way I think about it is, you start out with the units M/s for a zero-order reaction and then you keep dividing by M for each reaction after. For instance, the units for a first-order reaction are 1/s and the units for a second-order reaction are 1/M*s. Hence, each time you divide by M to get the units of the next order.
-
Tanmay Singhal 1H
- Posts: 143
- Joined: Sat Jul 20, 2019 12:16 am
-
Madelyn Romberg 1H
- Posts: 102
- Joined: Tue Oct 02, 2018 12:16 am
Re: Finding out order
Units correlate to the reaction order to ensure the final units work out. Hence, the units for the rate constant will indicate the rate order.
-
CalvinTNguyen2D
- Posts: 102
- Joined: Thu Jul 25, 2019 12:16 am
Re: Finding out order
For each different order, the rate constant will have its own units. If you can determine the units that the constant has, you can deduce the reaction order.
-
Joshua Swift
- Posts: 100
- Joined: Wed Sep 30, 2020 9:50 pm
Re: Finding out order
Each order has different units so you could potentially look at them to determine what order the reaction is.
-
Karina Grover 1A
- Posts: 101
- Joined: Wed Sep 30, 2020 9:34 pm
- Been upvoted: 1 time
Re: Finding out order
Yes, units are definitely a good way to determine order! The units of the rate constant will always ensure that, when multiplied by the according concentration units, the overall (rate) units are M/s or mol/Ls.
-
Tanner Bartyczak 1K
- Posts: 100
- Joined: Wed Sep 30, 2020 9:51 pm
-
Shrinidhy Srinivas 3L
- Posts: 121
- Joined: Wed Sep 30, 2020 9:39 pm
- Been upvoted: 2 times
Re: Finding out order
The units of a reactant or reaction can certainly be used to find the order. You would have to base it on the exponent of moles and liters, as this will help you determine order. I hope this helps!
-
Sai Ramadas 1J
- Posts: 50
- Joined: Thu Dec 17, 2020 12:18 am
Re: Finding out order
Yes, you can definitely use the units of the rate constant in the rate law to identify the order of the reaction, as others said. To add on, you can also use them the other way. If you know what the order of the reaction is and you are calculating the rate constant, you can make sure that you calculated it correctly by checking to see if the units are what they should be according to the order.
-
Gustavo_Chavez_1K
- Posts: 101
- Joined: Wed Sep 30, 2020 9:46 pm
Re: Finding out order
Yes, the constant k has different units for each order. The zeroth order has the units of M/s, the first order has the units 1/s, and the second order has the units of 1/M*s. With this, if we know the units of the k of the rate law then we can figure out which order each reagent is in.
-
Edgar Velazquez 2K
- Posts: 50
- Joined: Wed Nov 20, 2019 12:20 am
Re: Finding out order
Yes, you can use units to identify the rate orders since they all have different units. The zero order units are M/s. The first order units are 1/s. The second order units are 1/M x s.
-
Jacob_Eberson_2D
- Posts: 50
- Joined: Mon Jan 03, 2022 8:40 pm
Re: Finding out order
yes, you can match/cancel out units in the rate constant with units in the rate law
-
Saatvika Nair
- Posts: 49
- Joined: Wed Feb 17, 2021 12:17 am
Re: Finding out order
Yes, the units can be used - I believe zero order is m/s, first order is 1/s, and second order is 1/m * s. If you use the formulas to deduce what units the order should have and what is should look like, you should be good to go!
-
Konmal Ali 1G
- Posts: 107
- Joined: Fri Sep 24, 2021 6:38 am
Re: Finding out order
Because the units of the rate constant must cancel out with the units of the rate law, the units of the rate constant are reflective of the order of the reaction.
Return to “Second Order Reactions”
Who is online
Users browsing this forum: No registered users and 3 guests

