Hi everyone! Does anyone know how to determine k for a second order and third order reaction. The question is:
For each reaction order, identify the proper units for the rate constant, k. Not all of the choices will be used.
Answer choices: M/s, 1/s, M2/s, 1/(M2*s), 1/(M*s)
Sapling #4 WK 9/10
Moderators: Chem_Mod, Chem_Admin
-
Melody Haratian 2J
- Posts: 125
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 2 times
-
Marcus Lagman 2A
- Posts: 101
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 2 times
Re: Sapling #4 WK 9/10
Hello!
So as you learned, we graph the rate of the reaction by concentration vs. time. The unit for concentration is molarity (M) and for time is in seconds (s). Therefore, the rate reaction is in molarity per second (rate = M/s).
As a result, it is simply replacing parts of the formula of each rate reaction order with the corresponding units:
Zero Order: Rate = k so k = M/s
First Order: Rate = k [A] so k = 1/s
Second Order: Rate = k [A]^2 so k = 1/(M*s)
Third Order: Rate = k[A]^3 so k = 1/(M^2*s)
I hope this helps!
So as you learned, we graph the rate of the reaction by concentration vs. time. The unit for concentration is molarity (M) and for time is in seconds (s). Therefore, the rate reaction is in molarity per second (rate = M/s).
As a result, it is simply replacing parts of the formula of each rate reaction order with the corresponding units:
Zero Order: Rate = k so k = M/s
First Order: Rate = k [A] so k = 1/s
Second Order: Rate = k [A]^2 so k = 1/(M*s)
Third Order: Rate = k[A]^3 so k = 1/(M^2*s)
I hope this helps!
-
IsaacLaw1E
- Posts: 106
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 1 time
Re: Sapling #4 WK 9/10
One of the easiest ways it to use the equations for the half-times. All you need to know is that the units of [A] is M and the units for time is seconds.
For instance, the half-time equation of a first-order reaction is t=0.693/k. Solving for k = 0.693/t, and the units for this is 1/s.
For a second-order reaction, t=1/(k[A]). Solving for k = 1/(t[A]), and the units are 1/(M * s).
For a zero-order reaction, t = [A]/(2k). Solving for k = [A]/(2t), and the units are M/s.
From here, the pattern becomes clear. The units for k for a nth-order reaction is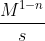 , where n is the order.
, where n is the order.
So for the third order, the units are M^(1-3)/s = M^(-2)/s = 1/(M^2 * s)
For instance, the half-time equation of a first-order reaction is t=0.693/k. Solving for k = 0.693/t, and the units for this is 1/s.
For a second-order reaction, t=1/(k[A]). Solving for k = 1/(t[A]), and the units are 1/(M * s).
For a zero-order reaction, t = [A]/(2k). Solving for k = [A]/(2t), and the units are M/s.
From here, the pattern becomes clear. The units for k for a nth-order reaction is
So for the third order, the units are M^(1-3)/s = M^(-2)/s = 1/(M^2 * s)
-
Eve Gross-Sable 1B
- Posts: 102
- Joined: Wed Sep 30, 2020 10:02 pm
- Been upvoted: 1 time
Re: Sapling #4 WK 9/10
Someone also explained it to me that you want to look at k as the factor that will be used to cancel out any of the units that are skewed by the order of the reaction so that the final answer will always be M/s. So whenever you are given one of these, it's helpful to look at what units are present and what they must be multiplied by (k) in order to cancel out any excess units to give you a final rate that has units in M/s.
-
Leyla Anwar 3B
- Posts: 101
- Joined: Wed Sep 30, 2020 10:03 pm
Re: Sapling #4 WK 9/10
The easiest way for me to remember the units is to think about the graphs Dr. Lavelle would draw in class.
For first order you plot ln[A] vs. time so the units will be 1/s.
For second order you plot 1/[A] vs. time so the units are 1/M*s.
For 0 order you plot [A] vs. time so the units are M/s.
For third order we haven't learned the graph yet but you can infer based on the second order that it would be 1/M^2*s
For first order you plot ln[A] vs. time so the units will be 1/s.
For second order you plot 1/[A] vs. time so the units are 1/M*s.
For 0 order you plot [A] vs. time so the units are M/s.
For third order we haven't learned the graph yet but you can infer based on the second order that it would be 1/M^2*s
-
RitaThomas_3G
- Posts: 100
- Joined: Wed Sep 30, 2020 9:40 pm
Re: Sapling #4 WK 9/10
For second order, the rate = k[A]^2. The units for each respective part are (M/s) = kM^2. Solve for k, and you get k = (1/M*s).
For third order, the rate = k[A]^3, The units for each respective part are (M/s) = kM^3. Solve for k, and you get k = (1/M^2*s).
For third order, the rate = k[A]^3, The units for each respective part are (M/s) = kM^3. Solve for k, and you get k = (1/M^2*s).
-
Emmaplant1c
- Posts: 49
- Joined: Wed Feb 17, 2021 12:17 am
Re: Sapling #4 WK 9/10
Marcus Lagman 2A wrote:Hello!
So as you learned, we graph the rate of the reaction by concentration vs. time. The unit for concentration is molarity (M) and for time is in seconds (s). Therefore, the rate reaction is in molarity per second (rate = M/s).
As a result, it is simply replacing parts of the formula of each rate reaction order with the corresponding units:
Zero Order: Rate = k so k = M/s
First Order: Rate = k [A] so k = 1/s
Second Order: Rate = k [A]^2 so k = 1/(M*s)
Third Order: Rate = k[A]^3 so k = 1/(M^2*s)
I hope this helps!
This was super helpful, I wasn't sure why 1/s would be first order instead of zero order!
Re: Sapling #4 WK 9/10
Zero Order: k = M/s
First Order: k = 1/s
Second Order: k = 1/(M*s)
Third Order: Rate = k= 1/(M^2*s)
First Order: k = 1/s
Second Order: k = 1/(M*s)
Third Order: Rate = k= 1/(M^2*s)
-
Michael Crannell 1H
- Posts: 102
- Joined: Fri Sep 24, 2021 6:39 am
-
Alice Guey 1B
- Posts: 100
- Joined: Fri Sep 24, 2021 7:35 am
Re: Sapling #4 WK 9/10
Michael Crannell 1H wrote:Where do you place M^2 / s, wouldn't it be a "negative 1" order technically.
For the purposes of the question, we are not required to pair M^2/s with a reaction order, but I suppose you would be correct. Some real reactions have negative orders, meaning that an increase in concentration of reactants is associated with a decrease in rate.
Return to “Second Order Reactions”
Who is online
Users browsing this forum: No registered users and 5 guests

