Hello, I am having trouble with the textbook problem 7B.13.
"The half-life of A in a second-order reaction is 50.5 s when
[A]0=0.84 mol/L. Calculate the time needed for the concentration of A to decrease to (a) one-sixteenth; (b) one-fourth; (c) onefifth of its original value."
I understand that the half-life equation for second-order reactions should be used to find k and the integrated rate law to find the time needed for each concentration. However, I am still having trouble figuring out this problem. Could someone help?
Thank you!
Textbook Problem 7B.13
Moderators: Chem_Mod, Chem_Admin
-
derickngo3d
- Posts: 111
- Joined: Wed Sep 30, 2020 9:51 pm
Re: Textbook Problem 7B.13
For a and b, you can simply use the fact that they are exponentials of the half life, which is 1/2 of the value of the initial concentration. So 1/16 would be (1/2)^4. So multiply the half-life in seconds by 4.
Hope this helps!
Hope this helps!
-
Christine Ma 3L
- Posts: 102
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Textbook Problem 7B.13
You're pretty much on the right track!
We have the half-life equation for a second-order reaction: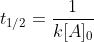
and its integrated rate law: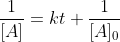 which can be rearranged into
which can be rearranged into 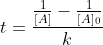 since we're only interested in time.
since we're only interested in time.
First we use the half-life equation to solve for k:
k=(0.84M)}=0.024M^{-1}s^{-1})
For part a, [A] should be equal to 1/16 of [A]0, and after substituting we should get:
\frac1{[A]_0}-\frac1{[A]_0}}{k}=\frac{\frac{16}{[A]_0}-\frac1{[A]_0}}{k}=\frac{\frac{15}{[A]_0}}{k})
(0.84M)}=7.4\cdot 10^2s) .
.
You solve parts b and c the same way, but for part b [A] should equal 1/4 of [A]0 and for part c [A] should equal 1/5 of [A]0.
Hope this helps!
We have the half-life equation for a second-order reaction:
and its integrated rate law:
First we use the half-life equation to solve for k:
k=
For part a, [A] should be equal to 1/16 of [A]0, and after substituting we should get:
You solve parts b and c the same way, but for part b [A] should equal 1/4 of [A]0 and for part c [A] should equal 1/5 of [A]0.
Hope this helps!
-
Zoe Kaiser
- Posts: 86
- Joined: Fri Sep 29, 2023 11:47 am
Re: Textbook Problem 7B.13
I get how it's solved but why do we have to solve it this way when in #7 we could just multiply by the half life?
Return to “Second Order Reactions”
Who is online
Users browsing this forum: No registered users and 4 guests

