Solving Equations using E=hv
Moderators: Chem_Mod, Chem_Admin
-
Lusin_Yengibaryan_3B
- Posts: 113
- Joined: Wed Sep 30, 2020 9:33 pm
Solving Equations using E=hv
How would you go about finding the frequency of a mole of photons given the energy of the said mole of photons?
-
Stuti Pradhan 2J
- Posts: 173
- Joined: Wed Sep 30, 2020 9:32 pm
- Been upvoted: 5 times
Re: Solving Equations using E=hv
I would assume that you could use 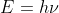 as you would with the frequency of a single photon because the frequency per photon gives you the energy per photon, so the frequency per mole should give you the energy per mole. The Js^-1 unit for Planck's constant should cancel with the J for the energy, and the s^-1 should cancel with the s^-1 of the frequency. That leaves the moles^-1 (per mole) unit from the energy and the frequency which will also cancel with each other. So you should be able to divide the given energy per mole of photons by Planck's constant to get the frequency per mole of photons.
as you would with the frequency of a single photon because the frequency per photon gives you the energy per photon, so the frequency per mole should give you the energy per mole. The Js^-1 unit for Planck's constant should cancel with the J for the energy, and the s^-1 should cancel with the s^-1 of the frequency. That leaves the moles^-1 (per mole) unit from the energy and the frequency which will also cancel with each other. So you should be able to divide the given energy per mole of photons by Planck's constant to get the frequency per mole of photons.
Hope this helps!
Hope this helps!
-
Lung Sheng Liang 3J
- Posts: 100
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Solving Equations using E=hv
Hello,
To find the frequency of photons you would generally use E=hv or C=lambda(v). Frequencies of one photon and one mole of photons are the same. To convert moles into number of photons or number of photons into moles just use Avogadro's constant.
To find the frequency of photons you would generally use E=hv or C=lambda(v). Frequencies of one photon and one mole of photons are the same. To convert moles into number of photons or number of photons into moles just use Avogadro's constant.
-
Jacob Schwarz-Discussion 3I
- Posts: 111
- Joined: Wed Sep 30, 2020 10:01 pm
Re: Solving Equations using E=hv
You would solve normally using E=hv and then divide by Avogadro's constant
-
Mauricio Maravilla 3C
- Posts: 50
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Solving Equations using E=hv
Just divide the total energy by Avogadro's number since you can assume all of the photons have the same frequency. I'm guessing the problem is talking about intensity, otherwise it wouldn't make sense to have a mole of photons. But after this it's just a simple plug and chug Planck's Constant and you can isolate the frequency of each photon.
-
Ansh Patel 2I
- Posts: 103
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Solving Equations using E=hv
Hi! To solve for the frequency of a mole of photons, work out the E=hv equation normally and then divide by Avogadro's constant.
-
Bai Rong Lin 2K
- Posts: 101
- Joined: Wed Sep 30, 2020 9:54 pm
Re: Solving Equations using E=hv
You would use E=hv to solve and then divide with avogadro's constant
Who is online
Users browsing this forum: No registered users and 2 guests

