During discussion section we were presented with this problem
We have a frequency of v= 8.7x 10^14 Hz what is the energy? So we would need to first find the wavelength using the formula wavelength x frequency = velocity. Then rearranging the equation so that we can find the wavelength to plug that in to find energy. So velocity/frequency= Wavelength which would be
3.00 x 10^8 ms^-1/ 8.7 x 10^14 Hzs^-1=3.45 x 10^-7
Know that we have the wavelength then we plug it in to the equation but which equation would we use I believe that we were supposed to use E=plancks constant x frequency/ wavelength
Is that not correct? Why not?
Discussion Section Problem
Moderators: Chem_Mod, Chem_Admin
-
Julia Meno 1D
- Posts: 28
- Joined: Fri Sep 29, 2017 7:04 am
Re: Discussion Section Problem
I think if you are given the frequency v, you can just use the equation E = hv, which would just be Planck's constant (h) multiplied by 8.7x 10^14 Hz. I don't think you'd even have to do the extra work to find the wavelength but maybe I'm misunderstanding!
-
Karan Singh Lecture 3
- Posts: 23
- Joined: Fri Sep 29, 2017 7:07 am
Re: Discussion Section Problem
I believe Julia is correct. We can simply plug the frequency in to the equation E=hv.
-
Asia Yamada 2B
- Posts: 102
- Joined: Wed Sep 30, 2020 9:36 pm
Re: Discussion Section Problem
When you are given the frequency and are asked to find the energy, all you have to do is plug that frequency into the equation, E=hv, where h (Planck’s constant) = 6.63 x 10^-34. When you solved for wavelength and simplified the equation, it resulted in E=h(v/wavelength) which is untrue.
-
Sarah Hong 2K
- Posts: 102
- Joined: Fri Sep 24, 2021 5:48 am
Re: Discussion Section Problem
You are already given the frequency which is 8.7*10^14 Hz. So you just have to plug in the correct values for the equation E=hV. So set h equal to 6.626*10^-34 J.s and V to 8.7*10^14 Hz. Multiply those two numbers together and you will get 5.76*10^-19.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Discussion Section Problem
Hello Allyson,
Please refer to the following equation for the energy quantity: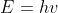 .
.
While the conversions you provided do offer extra practice for yourself, the equation above would be slightly more efficient. Happy to see all engaging in the discussion!
Please refer to the following equation for the energy quantity:
While the conversions you provided do offer extra practice for yourself, the equation above would be slightly more efficient. Happy to see all engaging in the discussion!
-
Ziyi Meng 2K
- Posts: 116
- Joined: Fri Sep 24, 2021 6:48 am
Re: Discussion Section Problem
I think you were over-thinking this problem. Since you are given the frequency, you can simply plug it into the E = hv equation to get the energy. Finding the wavelength would be unnecessary work because at the end of the day, you really only need the Planck's constant and the frequency to solve for energy.
-
LukeYing_3H
- Posts: 103
- Joined: Fri Sep 24, 2021 6:16 am
Re: Discussion Section Problem
You have frequency already so just plug it into E=hv with Plank's Constant.
-
Jonathan Liu 2I
- Posts: 112
- Joined: Fri Sep 24, 2021 6:39 am
Re: Discussion Section Problem
I think a more straightforward approach would be to use the equation E=hv and use the frequency given the plancks constant to find the energy.
Who is online
Users browsing this forum: No registered users and 7 guests

