Hi guys! I have been having o much trouble with this question. Please help
The electron in a hydrogen atom is excited to the n=6 shell and emits electromagnetic radiation when returning to lower energy levels. Determine the number of spectral lines that could appear when this electron returns to the lower energy levels, as well as the wavelength range in nanometers.
wavelength range from: _______ nm to ______ nm
I was about to get the spectral lines but not the range. For the first one I was able to get a frequency of 3.19 x 10 15. Then, I divided 3.0 x 108 by 3.19 x 10 15 to get 9.4 x 10 -8. Then I turn it into nm and get 9.4 x 10 -17. For the second one I get v = 4.02 x1013. Then, 3.0 x 108 divided by 4.02 x1013 and I get 7.46 x 10 -6 turning it into nm and get 7.46 x 10 -15. I keep getting this answer and keep getting it wrong can someone help me please. Thank you
Sapling Q. 6
Moderators: Chem_Mod, Chem_Admin
-
Anh Trinh 1J
- Posts: 156
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Sapling Q. 6
I think when you tried to convert the wavelength from meter to nm, you didn’t use the correct conversion. 1 nm = 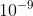 m.
m.
So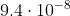 m converted to nm would be 94.
m converted to nm would be 94.
Same thing for the second one.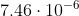 m converted to nm would be 7460 nm.
m converted to nm would be 7460 nm.
The wavelength range would then be from 94 nm to 7460 nm.
So
Same thing for the second one.
The wavelength range would then be from 94 nm to 7460 nm.
Last edited by Anh Trinh 1J on Fri Oct 23, 2020 8:10 pm, edited 1 time in total.
-
Jared Limqueco 3E
- Posts: 97
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Sapling Q. 6
I calculated the wl in meters, and you got those right. But you just got the conversion wrong. When converting m to nm, you don't divide by 10^9 (what you did), you multiply the number in meters by 10^9. Intuitively, you should have a bigger number in nm than in m. Hope this resolves it
-
Lesly Lopez 3A
- Posts: 115
- Joined: Wed Sep 30, 2020 9:54 pm
- Been upvoted: 1 time
Re: Sapling Q. 6
Anh Trinh 1B wrote:I think when you tried to convert the wavelength from meter to nm, you didn’t use the correct conversion. 1 nm =m.
Som converted to nm would be 94.
Same thing for the second one.m converted to nm would be 7460 nm.
The wavelength range would then be from 94 nm to 7460 nm.
Thank you!! Yeah me conversion was way off. Ooof you saved me lol thx.
-
Lesly Lopez 3A
- Posts: 115
- Joined: Wed Sep 30, 2020 9:54 pm
- Been upvoted: 1 time
Re: Sapling Q. 6
Jared Limqueco 1H wrote:I calculated the wl in meters, and you got those right. But you just got the conversion wrong. When converting m to nm, you don't divide by 10^9 (what you did), you multiply the number in meters by 10^9. Intuitively, you should have a bigger number in nm than in m. Hope this resolves it
Thank you for the help. I have no idea why I was dividing. Thank you!!
-
Rylee Mangan 1K
- Posts: 102
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 2 times
Re: Sapling Q. 6
I cannot figure out how to determine the number of spectral for question #6? Is there a different formula to use other than En=-hR/n^2 or DeltaE=Ef-Ei?
-
Lesly Lopez 3A
- Posts: 115
- Joined: Wed Sep 30, 2020 9:54 pm
- Been upvoted: 1 time
Re: Sapling Q. 6
Rylee Mangan 2B wrote:I cannot figure out how to determine the number of spectral for question #6? Is there a different formula to use other than En=-hR/n^2 or DeltaE=Ef-Ei?
Hi, for that it is simply 5. Reason being you got form 6-1, 6-2, 6-3, 6-4, and 6-5. Or the other way around. In that case there are just 5 spectral lines.
Re: Sapling Q. 6
Since it is excited to n=6 that means it start either from n =1, 2, 3, 4, or 5. This then means that there will be 5 spectral lines since there are only 5 energy levels to start from. To find the max you would have to start from the highest starting energy level (n=5) to n=6. Use the formula E = -hR/n^2 to do so. To find the min you would start from the lowest starting energy level (n=1) to n=6. Use the same formula (E = -hR/n^2) to do so. This question is looking for the range.
Who is online
Users browsing this forum: No registered users and 4 guests

