the problem states:
"As you may well know, placing metal objects inside a microwave oven can generate sparks. Two of your friends are arguing over the cause of the sparking, with one stating that the microwaves "herd" electrons into "pointy" areas of the metal object, from which the electrons jump from one part of the object to another. The other friend says that the sparks are caused by the photoelectric effect. Prove or disprove the latter idea using basic physics.
Suppose the typical work function of the metal is roughly 4.540×10−19 J. Calculate the maximum wavelength in angstroms of the radiation that will eject electrons from the metal"
I've been stuck with this problem for hours. please help!
Sapling HW #7
Moderators: Chem_Mod, Chem_Admin
-
Sarah_Hoffman_2H
- Posts: 104
- Joined: Wed Sep 30, 2020 9:54 pm
Re: Sapling HW #7
Using the work function, plug it into the energy value in the E=hc/lambda equation, and solve for wavelength. This will solve for maximum wavelength because it is using the absolute least amount of energy to eject an electron. Basically the top paragraph is just filler/unnecessary information.
-
Sami Siddiqui 1J
- Posts: 165
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 6 times
Re: Sapling HW #7
To solve for this question, consider one thing when it comes to ejecting electrons:
energy of the incoming photon = energy needed to remove electron (work function) + kinetic energy
If we want to maximize the wavelength, we would need to set the kinetic energy equal to 0 because we want to know what wavelength of light can barely remove the electron from the atom itself. As you may already know, the longer the wavelength, the less energy the photon possesses so there's a certain maximum wavelength we can have before there's insufficient energy to remove the electron.
Knowing this,
energy of the photon (hv) = work function
From there, you can actually substitute v for c/wavelength (recall c = wavelength * v) into the left side of the energy equation:
(hc)/wavelength = work function
This should help you find the wavelength. Regarding the next part in which you're likely given a light of a random frequency (in GHz if I remember correctly) and it'll ask you if that light is sufficient in removing electrons in the microwave situation. This can approached in one of two ways: 1) use E = hv for the frequency you're given and see if it's greater than the work function or 2) use c = v * wavelength and see if the wavelength you find here is greater than the maximum wavelength you found in the previous question. If the energy you find using the first method does not exceed the work function, no electrons are ejected; if the wavelength you find using the second method is greater than the max wavelength for ejecting electrons, then no electrons will be ejected there as well.
energy of the incoming photon = energy needed to remove electron (work function) + kinetic energy
If we want to maximize the wavelength, we would need to set the kinetic energy equal to 0 because we want to know what wavelength of light can barely remove the electron from the atom itself. As you may already know, the longer the wavelength, the less energy the photon possesses so there's a certain maximum wavelength we can have before there's insufficient energy to remove the electron.
Knowing this,
energy of the photon (hv) = work function
From there, you can actually substitute v for c/wavelength (recall c = wavelength * v) into the left side of the energy equation:
(hc)/wavelength = work function
This should help you find the wavelength. Regarding the next part in which you're likely given a light of a random frequency (in GHz if I remember correctly) and it'll ask you if that light is sufficient in removing electrons in the microwave situation. This can approached in one of two ways: 1) use E = hv for the frequency you're given and see if it's greater than the work function or 2) use c = v * wavelength and see if the wavelength you find here is greater than the maximum wavelength you found in the previous question. If the energy you find using the first method does not exceed the work function, no electrons are ejected; if the wavelength you find using the second method is greater than the max wavelength for ejecting electrons, then no electrons will be ejected there as well.
-
Isabella Chou 1A
- Posts: 110
- Joined: Wed Sep 30, 2020 9:52 pm
- Been upvoted: 1 time
Re: Sapling HW #7
From the photoelectric effect experiment, we know that  = E_{k}) . Since you are calculating the maximum or longest wavelength possible that will eject electrons, you can assume that the kinetic energy is 0, so
. Since you are calculating the maximum or longest wavelength possible that will eject electrons, you can assume that the kinetic energy is 0, so 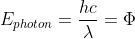 , and you solve for the wavelength. In the second part of the problem, you would find the minimum frequency of the incident light required to eject electrons (frequency corresponding to the wavelength you calculated previously) and compare this frequency to the given frequency. I hope this helps!
, and you solve for the wavelength. In the second part of the problem, you would find the minimum frequency of the incident light required to eject electrons (frequency corresponding to the wavelength you calculated previously) and compare this frequency to the given frequency. I hope this helps!
-
Francesca_Borchardt_2D
- Posts: 109
- Joined: Wed Sep 30, 2020 9:38 pm
Re: Sapling HW #7
In addition to what everyone else said, make sure that you convert your wavelength from m to angstroms. (1 angstrom=10^-10m). To do this, you take your wavelength in meters / conversion factor (10^-10).
Hope this helps!
Hope this helps!
-
Emma Chang 1G
- Posts: 52
- Joined: Wed Sep 30, 2020 10:02 pm
Re: Sapling HW #7
The energy of the incoming photon must be greater than or equal to the work function in order to eject electrons. Therefore, the energy of the incoming photon must be at least 4.540×10−19 J. You would use this in the equation E = (hc)/λ, or rearrange it to λ = (hc)/E, to solve for the minimum wavelength. So you would have λ = (6.626e-34 J.s x 3e8m.s^-1)/4.540×10−19 J, and then convert this from meters to angstroms. Hope this helped!
-
Charisma Arreola 2F
- Posts: 106
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Sapling HW #7
So you will need to compute using E=frequency x wavelength and use that to complete the kinetic energy equation.
-
Kaitlyn Hernandez 3I
- Posts: 143
- Joined: Wed Sep 30, 2020 9:37 pm
- Been upvoted: 2 times
Re: Sapling HW #7
When you are given energy and you have to solve for wavelength, you can use the equation 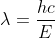 . You are already given energy, so you would then solve for wavelength in meters. Then you can convert that wavelength that you got into angstroms by using 1 A = 10^-10 m :)
. You are already given energy, so you would then solve for wavelength in meters. Then you can convert that wavelength that you got into angstroms by using 1 A = 10^-10 m :)
Who is online
Users browsing this forum: No registered users and 2 guests

