work function
Moderators: Chem_Mod, Chem_Admin
-
Hannah Kaye 1L
- Posts: 108
- Joined: Fri Sep 24, 2021 5:55 am
Re: work function
So my understanding is that the work function is simply threshold energy for the photoelectron effect equation. So to find it you would need to figure out the energy of the light or photon that is shining on the metal and the kinetic energy of the electron that is coming off of the metal. E(Photon)-Kinetic Energy=Work Function is the equation I would use.
-
Minoo Bastani 2J
- Posts: 103
- Joined: Fri Sep 24, 2021 6:51 am
Re: work function
The definition of the work function that we're using is the minimum amount of energy that a photon needs to have in order to force the metal/atom to eject an electron. I believe Dr. Lavelle also used "threshold energy" as an alternate term because it represents the threshold of energy that needs to be reached. We can solve for it by using:
Kinetic Energy= (Energy per photon) - (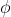 (work function)
(work function)
And rearranging it algebraically to solve for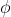 if you have the other two values.
if you have the other two values.
Kinetic Energy= (Energy per photon) - (
And rearranging it algebraically to solve for
-
Reagan Feldman 1D
- Posts: 102
- Joined: Fri Sep 24, 2021 5:44 am
- Been upvoted: 2 times
Re: work function
As mentioned in the previous responses, the work function,(also referred to as the threshold energy) as it relates to the photoelectric effect, is the minimum amount of energy required to remove/eject electrons from a metal surface. We can solve for work function by rearranging the equation E(photon)-E(energy remove e-) = Ek(e-).
-
Andrew Nguyen 1E
- Posts: 101
- Joined: Fri Sep 24, 2021 6:34 am
- Been upvoted: 1 time
Re: work function
Hello,
The work function is the amount of energy required to remove an electron from the surface of a metal. It is also known as the threshold energy. To calculate it, you would need the kinetic energy of the released electron and the energy/wavelength/frequency of the incoming photon. Since E(incoming photon) - work function = kinetic energy of released electron, you would rearrange the equation with the variables you have to solve for the work function.
The work function is the amount of energy required to remove an electron from the surface of a metal. It is also known as the threshold energy. To calculate it, you would need the kinetic energy of the released electron and the energy/wavelength/frequency of the incoming photon. Since E(incoming photon) - work function = kinetic energy of released electron, you would rearrange the equation with the variables you have to solve for the work function.
-
Irene Kim 3E
- Posts: 106
- Joined: Fri Sep 24, 2021 6:49 am
Re: work function
Hi!
To add to previous replies, the value of the work function differs depending on the atom and that particular electron's movement. The equation used is E(photon) - work function/threshold energy = kinetic energy (electron). The energy of the photon can be derived using the equation(s) E = h*v and c = wavelength*v (where v is frequency, not velocity). These equations can be rearranged to give E = (hc)/wavelength. The kinetic energy can be derived using the equation 1/2(mv2), where m and v refer to the mass and velocity of the electron that was ejected from the metal.
Now that you have both the energy of the photon and the kinetic energy of the electron, you can use the first equation to solve for the work function. Hope this was helpful!
To add to previous replies, the value of the work function differs depending on the atom and that particular electron's movement. The equation used is E(photon) - work function/threshold energy = kinetic energy (electron). The energy of the photon can be derived using the equation(s) E = h*v and c = wavelength*v (where v is frequency, not velocity). These equations can be rearranged to give E = (hc)/wavelength. The kinetic energy can be derived using the equation 1/2(mv2), where m and v refer to the mass and velocity of the electron that was ejected from the metal.
Now that you have both the energy of the photon and the kinetic energy of the electron, you can use the first equation to solve for the work function. Hope this was helpful!
-
Aryan Gajjar 3D
- Posts: 114
- Joined: Fri Sep 24, 2021 6:05 am
- Been upvoted: 1 time
Re: work function
Work function is the minimum amount of energy required to eject an electron from the surface of the metal.
-
Nico Towfighian 3L
- Posts: 76
- Joined: Fri Sep 24, 2021 5:49 am
Re: work function
The work function looks like the letter o with a line going down the middle, and all it means is the amount of work, or energy, required for a photon to have in order to be sufficient enough to excite the electron of the atom into a high enough state that emits the electron off the surface of such metal. It really isn't something too much on its own, but in problems solving for it can be done knowing the kinetic energy of the emitted electron, and the energy of the incoming photon.
-
Eric Sun 2G
- Posts: 102
- Joined: Fri Sep 24, 2021 5:08 am
Re: work function
The work function is also known as the threshold energy, which is the minimum energy required to excite an electron to another energy level.
-
Shiley_David_1D
- Posts: 53
- Joined: Fri Sep 24, 2021 6:04 am
Re: work function
Hannah Kaye 3E wrote:can anyone help explain what the work function is and how to solve for it?
Hello Hannah!
The work function is defined as the minimum amount of energy required to remove an electron from a surface, in the example in the class it was a metal surface, to a point in the vacuum outside of the surface. Another way to refer to it is the threshold energy. The equation I wrote down in my notes relating to the work function is:
(E=hv) - φ(work function) = Ek = 1/2me-ve-^2
As for solving for the work function, you could probably utilize the values you are given, plug them into the equation, and manipulate the variables until you get the work function by itself. To be entirely honest, that previous sentence is based on pure guessing because the worked examples following the equations in the module for this topic provided the work function in the question, so I am not totally certain how to solve for it. If anyone does know, let me in on your knowledge please lol.
As a side notes:
-in my notes the notation is more of a sideways zero with a line through it, but when I copied and pasted the symbol it ended up like that lol
-k and e and supposed to be subscripts but I am uninformed as to how to type that in this forum, so if anyone knows please fill me in
-the experiment must be conducted in a vacuum otherwise molecules from the surrounding air, such as N2, O2, CO2, would collide with the electrons and mess up the experiment
This was sort of all over the place, but I hope this helps in some capacity!
-
Santiago Chang 2K
- Posts: 54
- Joined: Fri Sep 24, 2021 6:55 am
Re: work function
The work function is the minimum amount of energy required to eject a single electron. Subtracting that value from the energy of the proton allows you to determine the results. If the answer is negative, then no electrons are ejected. If the answer is 0, then electrons are ejected with no kinetic energy. If the answer is higher than 0, then the remaining energy is the amount of kinetic energy present in each electron. Hope this helped!!!
-
Chris Korban 1D
- Posts: 107
- Joined: Fri Sep 24, 2021 6:53 am
Re: work function
The work function is the required amount of energy needed to remove an electron from a metal surface using the photoelectric effect. It is also called the energy threshold and is calculated using equation: Work function= Energy of the photon- kinetic energy of the ejected electron.
-
Neelaj Das 3I
- Posts: 103
- Joined: Fri Sep 24, 2021 5:35 am
Re: work function
The work function is simply the minimum, required energy necessary to eject an electron from a metal surface. This is the threshold energy and if the light does not meet this minimum requirement, the electron won't be ejected.
-
Edwin Montalvo 1G
- Posts: 113
- Joined: Fri Sep 24, 2021 6:27 am
Re: work function
The work function is the amount of energy required to eject an electron from its metal surface, also known as the threshold energy. It can be calculated using this formula: E (photon)- E (kinetic energy) = work function.
Remember that all of these parts of the equation are in energy, which is typically in Joules (J). Sometimes, the work function is given in kJ/mole, signifying the amount of energy required to remove 1 mole of electrons. Don't forget to convert this back into Joules (J) in order to use it in the equation. Hope this helps!
Remember that all of these parts of the equation are in energy, which is typically in Joules (J). Sometimes, the work function is given in kJ/mole, signifying the amount of energy required to remove 1 mole of electrons. Don't forget to convert this back into Joules (J) in order to use it in the equation. Hope this helps!
-
Mahima Manoj 1F
- Posts: 50
- Joined: Fri Sep 24, 2021 6:09 am
Re: work function
Work function also called the threshold energy is the energy required to eject an electron from a metal surface. This can be solved for by the equation (E=hv) - Eκ - ½ m(sub e-) v(sub e-) ^2 = Φ (work function).
-
almaortega
- Posts: 104
- Joined: Fri Sep 24, 2021 6:54 am
Re: work function
So the work function is the greek letter phi= Kinetic Energy= 1/2(mass of electron)-velocity of electron^2, which is the threshold for the amount of energy needed to remove electrons. Sometimes the problem gives you some of the variables above, or you may have to rearrange the equation to solve for one variable needed for another equation. This equation is tied into E=hv.
-
amreen_sandhu1k
- Posts: 101
- Joined: Fri Sep 24, 2021 6:10 am
Re: work function
Everyone else's responses look correct but I also believe that the work fucntion is equal to the threshold frequency*planck's constant!
-
Joellen 1B
- Posts: 104
- Joined: Fri Sep 24, 2021 6:24 am
Re: work function
The work function is basically the amount of energy needed to eject an electron from a metal surface. to solve for it, you would use the equation E(proton)= (work function) + (1/2)(MV^2)
The (1/2)(MV^2) is also just kinetic energy. I would memorize this equation because it is not on the formula sheet.
The (1/2)(MV^2) is also just kinetic energy. I would memorize this equation because it is not on the formula sheet.
-
August Blum Dis 3D
- Posts: 54
- Joined: Fri Sep 24, 2021 5:30 am
Re: work function
The work function is equal to E(photon)-Kinetic energy(e). You can solve for it algebraically.
-
Simone Byun 1F
- Posts: 101
- Joined: Fri Sep 24, 2021 5:58 am
- Been upvoted: 1 time
Re: work function
The work function is the threshold energy for an electron or how much energy it'll take to remove an electron. It can be calculated as (work function) = E(photon) - kinetic energy.
-
Lawrence Javelo Disc 3B
- Posts: 77
- Joined: Fri Sep 24, 2021 6:43 am
Re: work function
Hi,
The work function (capital phi) is the energy required to eject an electron from the orbital of an atom. The equation Kinetic Energy = Energy of the Photon - Energy to remove electron (which is the work function). You can rearrange this equation to solve for it if it is not provided in the problem.
The work function (capital phi) is the energy required to eject an electron from the orbital of an atom. The equation Kinetic Energy = Energy of the Photon - Energy to remove electron (which is the work function). You can rearrange this equation to solve for it if it is not provided in the problem.
-
Emaad Sohail 3F
- Posts: 90
- Joined: Fri Sep 24, 2021 5:13 am
Re: work function
It's confusingly named, since it isn't a *function*, but the work function is the energy needed to remove a single electron from a metal via the photoelectric effect. It's equal to the energy of the photon emitted (Planck's constant times the frequency), minus the kinetic energy of the electron ejected (1/2 times the mass of the electron, times the velocity squared). I imagine it like, if you have an amount of energy in a photon, and you use some of it to eject an electron, the rest goes into the electron's movement.
-
Nomi Heidari-Bateni 2K
- Posts: 103
- Joined: Fri Sep 24, 2021 5:24 am
Re: work function
The work function describes the minimum energy required to remove an electron from the surface of a metal. It is solved using the equation: E(photon) - E(energy remove e-, aka work function) = E(excess) = EK(e-) = 0.5mv^2.
-
Molly McAndrew 1 1H
- Posts: 103
- Joined: Fri Sep 24, 2021 5:08 am
Re: work function
In one of the UA Step-Up sessions I attended, the UA mentioned that this equation involving the work function is only useful when dealing with photons of light. If a problem involves electrons or something else that has a defined mass, does this equation no longer apply at all?
Who is online
Users browsing this forum: No registered users and 5 guests

