Rate Law
Moderators: Chem_Mod, Chem_Admin
-
hannah_flagg_3F
- Posts: 10
- Joined: Wed Sep 21, 2016 2:57 pm
Rate Law
When a problem asks for the rate law of the overall reaction and you are given the slow step with a chemical reaction, a fast step with a chemical reaction, and the overall reaction, do you only write the rate law based off the slow step?
Re: Rate Law
I'm wondering this too. When do we have to use the pre-equilibrium approach and when can we just calculate the rate of the slowest step?
-
Annie Duong 3L
- Posts: 13
- Joined: Fri Jul 15, 2016 3:00 am
Re: Rate Law
Yes, to determine the rate determining step the slowest of the multiple steps is used to dictate the overall reaction rate.
For a proposed mechanism to be valid, the following conditions MUST be satisfied (no exceptions):
1. The elementary steps sum to the overall reaction.
2. The rate law of the slow step MUST agree with the experimentally determined rate law.
So for example:
 + NO_2(g) \rightarrow NO_3(g) + NO(g)) SLOW
SLOW
 + CO(g) \rightarrow NO_2(g) + CO_2(g)) FAST
FAST
 + CO(g) \rightarrow NO + CO_2(g))
If you add the Step 1 and Step 2 reactions together, you see that you will get the overall reaction, so this satisfies condition 1.
The experimentally determined rate law is (usually given):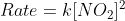
Now, if we write the out the rate law of the slow step we get which satisfies condition 2!
which satisfies condition 2!
However, remember that if your slow step contains an intermediate you cannot put the intermediate into your rate law because it will not be consistent with the experimentally determined rate law. When you find that there is an intermediate in the slow step, that is when you will use the pre-equilibrium approach!
For a proposed mechanism to be valid, the following conditions MUST be satisfied (no exceptions):
1. The elementary steps sum to the overall reaction.
2. The rate law of the slow step MUST agree with the experimentally determined rate law.
So for example:
If you add the Step 1 and Step 2 reactions together, you see that you will get the overall reaction, so this satisfies condition 1.
The experimentally determined rate law is (usually given):
Now, if we write the out the rate law of the slow step we get
However, remember that if your slow step contains an intermediate you cannot put the intermediate into your rate law because it will not be consistent with the experimentally determined rate law. When you find that there is an intermediate in the slow step, that is when you will use the pre-equilibrium approach!
Return to “Reaction Mechanisms, Reaction Profiles”
Who is online
Users browsing this forum: No registered users and 6 guests

