Units
Moderators: Chem_Mod, Chem_Admin
-
Erik Buetow 1F
- Posts: 96
- Joined: Fri Aug 30, 2019 12:15 am
Units
Will units for k values given a trial chart always be M^-2.s^-1? Or will this unit change based on the reaction order?
-
Ariana Iranmahboub1G
- Posts: 114
- Joined: Fri Aug 09, 2019 12:17 am
Re: Units
It would depend on the reaction order. For instance, if it is zero order the unit is mol*L^-1*s^-1. For the first order, it is s^-1. And for the second order, it is L*mol^-1*s^-1
-
JamieVu_2C
- Posts: 108
- Joined: Thu Jul 25, 2019 12:16 am
Re: Units
The units for k will depend on the overall order of the reaction. k has the units of L^2 * mol^-2 * s^-1 when the overall order is 3.
-
Julie Park 1G
- Posts: 100
- Joined: Thu Jul 25, 2019 12:15 am
Re: Units
You can use example 7A.7 from the book to see exactly the units for k change based on the reaction order! A easy way to find out the units is to use 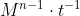 where M = mol/L and n is the reaction order
where M = mol/L and n is the reaction order
Return to “Reaction Mechanisms, Reaction Profiles”
Who is online
Users browsing this forum: No registered users and 4 guests

