The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction is reduced by 74.00 kJ⋅mol−1.
Uncatalyzed: A⟶B Ea=139.00 kJ⋅mol−1
Catalyzed: A⟶B Ea=65.00 kJ⋅mol−1
Determine the factor by which the catalyzed reaction is faster than the uncatalyzed reaction at 286.0 K if all other factors are equal.
Determine the factor by which the catalyzed reaction is faster than the uncatalyzed reaction at 344.0 K if all other factors are equal.
Can someone help me with this problem? What should I do first?
Sapling #20 (Week 9/10)
Moderators: Chem_Mod, Chem_Admin
-
Anthony_3C
- Posts: 89
- Joined: Wed Sep 30, 2020 10:00 pm
Re: Sapling #20 (Week 9/10)
You need the Arrhenius equation: k = Ae^(-Ea/RT) Plug in the Ea's to find the k of uncatalyzed and catalyzed reaction. Then plug in 286K in T and calculate k(cat)/k(uncat). Plug in 344 next. Also, remember to multiply Ea by 1000 because you need to convert kJ to J.
-
Amanda Bueno-Kling
- Posts: 111
- Joined: Wed Sep 30, 2020 9:45 pm
- Been upvoted: 2 times
Re: Sapling #20 (Week 9/10)
The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction is reduced by 74.00 kJ⋅mol−1.
Uncatalyzed: A⟶B Ea=139.00 kJ⋅mol−1
Catalyzed: A⟶B Ea=65.00 kJ⋅mol−1
Determine the factor by which the catalyzed reaction is faster than the uncatalyzed reaction at 286.0 K if all other factors are equal.
Determine the factor by which the catalyzed reaction is faster than the uncatalyzed reaction at 344.0 K if all other factors are equal.
I helped someone else get through this problem step by step, so I will explain it for you as well as it can be a bit complicated at first!
We will be using the formula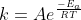
You can leave everything as is, but plug in the activation energy values we are given for Ea.
For k_uncat, we would have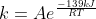
For k_cat, we would have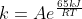
Then, we can divide them using K_cat/K_uncat.
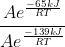
The A will cancel and we will be left with
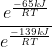
Using exponent rules, we can then subtract the exponents from each other.
Now we have:
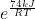
Now we convert KJ to J because R is in J. Now we have:
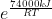
Then you just plug this into your calculator to get the answer, with e being Euler's constant, R being the gas constant (8.3145), and T being the temperature you are given, which here is 286.0 K for the first one, and 344.0 K for the second one.
Uncatalyzed: A⟶B Ea=139.00 kJ⋅mol−1
Catalyzed: A⟶B Ea=65.00 kJ⋅mol−1
Determine the factor by which the catalyzed reaction is faster than the uncatalyzed reaction at 286.0 K if all other factors are equal.
Determine the factor by which the catalyzed reaction is faster than the uncatalyzed reaction at 344.0 K if all other factors are equal.
I helped someone else get through this problem step by step, so I will explain it for you as well as it can be a bit complicated at first!
We will be using the formula
You can leave everything as is, but plug in the activation energy values we are given for Ea.
For k_uncat, we would have
For k_cat, we would have
Then, we can divide them using K_cat/K_uncat.
The A will cancel and we will be left with
Using exponent rules, we can then subtract the exponents from each other.
Now we have:
Now we convert KJ to J because R is in J. Now we have:
Then you just plug this into your calculator to get the answer, with e being Euler's constant, R being the gas constant (8.3145), and T being the temperature you are given, which here is 286.0 K for the first one, and 344.0 K for the second one.
Return to “Reaction Mechanisms, Reaction Profiles”
Who is online
Users browsing this forum: No registered users and 9 guests

