Adsorption
Moderators: Chem_Mod, Chem_Admin
-
Jack Papciak 2F
- Posts: 31
- Joined: Fri Sep 29, 2017 7:06 am
-
Masih Tazhibi 2I
- Posts: 33
- Joined: Fri Sep 29, 2017 7:06 am
Re: Adsorption
Adsorption occurs when you have a heterogenous catalyst being used, that is, a catalyst that is in a different phase than the reactants. Usually, adsorption occurs when your catalyst is a solid, and the reactants, rather than going inside or being absorbed by the catalyst, simply sit on top of it as the reaction proceeds.
-
lizzygaines1D
- Posts: 52
- Joined: Thu Jul 13, 2017 3:00 am
-
Emily Oren 3C
- Posts: 50
- Joined: Fri Sep 29, 2017 7:07 am
Re: Adsorption
Adsorption is when the catalyst is a solid and the reaction takes place on its surface. This image is an example to help visualize: 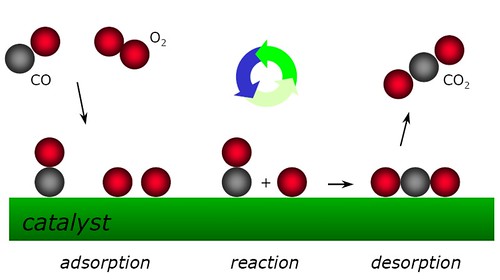

-
aaron tang 2K
- Posts: 49
- Joined: Thu Jul 27, 2017 3:01 am
Re: Adsorption
During lecture, Lavelle talked about how sponges absorb water and catalysts are adsorbing a reactant, meaning the reactant sits on top of the catalyst. There is a slight adhesion when a catalyst adsorbs a reactant. This allows the reactant to detach from the catalyst as the reaction proceeds. Once the detachment occurs, the catalyst is free to move onto the next reactant.
Return to “Arrhenius Equation, Activation Energies, Catalysts”
Who is online
Users browsing this forum: No registered users and 5 guests

