Page 1 of 2
MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Oct 26, 2018 9:58 am
by Chem_Mod
Eventually, my posts always get lost.
The title says "Garlic Bread" so that you can search "Garlic Bread" and easily find this post!
This is Lyndon Bui, a UA, and I have created many helpful practice problems for your midterm. The questions are modeled after previous test and exam questions, but it may not have everything you need to know. This covers material for the midterm.
Please complete these problems before my review session. My review session this quarter (F18) will be Saturday, Nov3 in CS24: 3-6PM I will go over each problem in detail during my review session. The answers, but not full solutions or work, will be posted here at this post after the review session. Any errors in the test will be posted below as well.
Printed copies will NOT be provided at the review session.
Link to Download Problems: Problems no longer available. Quarter has ended. See Course website and syllabus for most up-to-date material.
***This is not indicative of the structure, length, or format of the actual midterm. Treat these as extra practice problems. ***
Errors: None Found Yet (I'm too good)
Link for Answers: No Longer Available
Happy Studying and Good Luck!
-Lyndon Bui, UA
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Oct 26, 2018 10:00 am
by Phil Timoteo 1K
Thank you so much for the help!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Oct 28, 2018 2:15 pm
by Riya Shah 4H
Thank you so much! Are you going to post more midterm practice worksheets? Where can we get more practice problems from?
LYNDON: I will not be posting more practice worksheets. I do hand out paper copies of practice at my normal session workshops. However, you can only get previous ones from students who attended.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Oct 28, 2018 3:53 pm
by harperlacroix1a
Thank you!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Oct 28, 2018 3:55 pm
by ArielKim3C
Thank you so much for the extra practice!!!!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Mon Oct 29, 2018 4:33 pm
by Julia Lee
Hi, is the review session in Young Hall where our lectures are or is it CS24?
Re: MIDTERM PRACTICE - Garlic Bread Review Session [ENDORSED]
Posted: Mon Oct 29, 2018 6:58 pm
by ariana_apopei1K
Chan Mee Lee wrote:Hi, is the review session in Young Hall where our lectures are or is it CS24?
Hey, the session is in CS24, which is the same place where the 2 pm lecture is held. If yours is in CS50 it will be right next door!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Mon Oct 29, 2018 9:57 pm
by Liza Hayrapetyan-3K
Is this the only review session you will be having?
Lyndon: I have my regular UA sessions 6-8pm Tuesday night in Covel 210 where I review material from each week. This Saturday is my only midterm review session, but it is 3 hours and should be plenty!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Tue Oct 30, 2018 2:44 pm
by Kenan Kherallah 2C
Does anyone know how to do question 13 a and b? I though I could do 13a but thirteen b said that what she did (which is what I did) was wrong.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Thu Nov 01, 2018 12:23 pm
by Julia Jones 1G
Thanks for the midterm practice problems!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Thu Nov 01, 2018 10:03 pm
by 705192887
Can anyone help me with 9b? It asks for the electron configuration of Chromium. I got an answer of [Ar]4s2 3d4 but I looked it up online and it says that its' actually [Ar]4s1 3d5. Why is one of the 4s electrons actually in the 3d orbital? I looked it up and it mentioned something about stability? So does this always happen with elements in the 3d block or is it an exception for chromium? Thanks
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Thu Nov 01, 2018 11:07 pm
by Nicole Elhosni 2I
Chromium is an exception, because it will be more stable to have half of the d block filled and only one electron in the s block, [Ar]3d5 4s1 instead of [Ar]3d4 4s2. Copper is also an exception, and its electron configuration will be [Ar]3d10 4s1. From its placement on the periodic table, you would expect it to be [Ar]3d9 4s2 (like for Chromium you would expect [Ar]3d4 4s2) but it is more stable having an entirely full d block and one electron in its valence s shell.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Nov 02, 2018 12:54 am
by yaosamantha4F
Thanks for the practice midterm problems!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Nov 02, 2018 2:23 pm
by Karla_Ocampo 4E
Thank you very much!! This is so helpful
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Nov 02, 2018 2:30 pm
by Karla_Ocampo 4E
can anyone please help me with number 2? I got to dividing the molar mass of 667 by 30.024 and got 22? but i think its wrong because its such a large number. Thus my molecular formula so far is C22H44O22 ?? pls help
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Nov 02, 2018 2:42 pm
by Minsub Lee 3E
I got a molar mass of 30g/mol as well. I believe that the calculations are right.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Nov 02, 2018 5:00 pm
by Karla_Ocampo 4E
For number 6 part A, we need to use the DeBroglie wavelength equation right? I used it and got 1.5547 x 10^/34 m but im not sure if I used the right equation? and I also think I messed up the units so pls correct me if needed
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Fri Nov 02, 2018 5:06 pm
by Chem_Mod
Hello Everyone,
This is Lyndon! Regarding questions posted about the practice problems, don't worry! It will all be addressed tomorrow CS24 3-6pm (Saturday Nov 3).
After the review session, feel free to come talk to me to clarify anything you're not comfortable with!
See you tomorrow!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 8:16 am
by aisteles1G
Hello, I was just wondering if you will still be posting solutions online after the review session? On the practice midterm I know it says there won't be detailed solutions but the answers will still be posted? Thanks!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 12:04 pm
by Alex Alonso - 4F
Thank you so much!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 1:09 pm
by Brian Kwak 1D
thank you!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 4:19 pm
by DavidEcheverri3J
Karla_Ocampo 4E wrote:can anyone please help me with number 2? I got to dividing the molar mass of 667 by 30.024 and got 22? but i think its wrong because its such a large number. Thus my molecular formula so far is C22H44O22 ?? pls help
Same... :(
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 4:27 pm
by DavidEcheverri3J
DavidEcheverri3J wrote:Karla_Ocampo 4E wrote:can anyone please help me with number 2? I got to dividing the molar mass of 667 by 30.024 and got 22? but i think its wrong because its such a large number. Thus my molecular formula so far is C22H44O22 ?? pls help
Same... :(
Actually, I just solved it. ill walk you through it:
first you want ti divide the percentages by the molar mass. (C - 3.6 ; H - 6.3 ; O - 3.15). Next, you want to divide by the smallest number (3.15). (C - 1.14 ; H - 2; O - 1). Now, you want to multiply all the numbers until the one with a decimal becomes a whole number. So multiply by 7. You will be left with C8H14O7. After this, you find its molar mass (approx. 222 g/mol), and divide 667 by it. Then you multiply the empirical by 667/222 to get C24H42O21. Ah-hah!
Good luck!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 6:25 pm
by Daisylookinland
For #6, how are you supposed to convert the molar mass of GarBreadium to kg in order to use the de broglie equation?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 6:45 pm
by Kevin Tang 4L
Daisylookinland4B wrote:For #6, how are you supposed to convert the molar mass of GarBreadium to kg in order to use the de broglie equation?
You change it to kg you divide the molar mass of GarBreadium by Avogadro's constant, 6.02*10^23 which gives you 5.244*10^-24g then you divide again by 1000 to get the value in kg.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 8:18 pm
by Lisa Werner 2F
Thank you so much! This was really helpful!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 9:12 pm
by Elle_Mendelson_2K
Hello! Does anyone know where we can find the solutions sheet. Thank you!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 9:39 pm
by Angela Cong 3C
hello! does anyone know how soon the solutions sheet will be up?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 9:41 pm
by Elle_Mendelson_2K
Will someone explain 8b? I know that we need to use all of the information given in the question but I am not sure how wavelength corresponds to work functions. Thank you!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 10:11 pm
by Sarah Zhao 4C
Thank you Lyndon for pushing through today's review session. Feel better and good luck on your own midterms :)
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 10:29 pm
by paytonm1H
Thank you so much for all your help today Lyndon!! You are incredibly generous with your time! Good luck on your midterms and get well soon!!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 10:29 pm
by Javier_Ochoa_DIS_3J
Elle_Mendelson_4I wrote:Will someone explain 8b? I know that we need to use all of the information given in the question but I am not sure how wavelength corresponds to work functions. Thank you!
So the main equation you need is Eph = (work function of metal) - Ek (energy of electron ejected)
This is not correct. See post below.Everything has a wavelength and this can give you the energy for the work function equation.
The equation that you need to find Ek is ---> λ = h / m v
Input the wavelength into that equation 1.10 nm (must be in meters) and find the Velocity of the ejected electron.
So the equation EK = 1/2 m v^2 is used to find the energy of the ejected electron, Input the mass of electron and the velocity to find Ek
So now use Eph = (work function of metal) - Ek (energy of electron ejected)
Eph is the energy of the laser pointer.
Use E = h ν to to find frequency.
Frequency will be in hertz
Don't get mix up v and ν. One means frequency and the other means velocity. Idk you just have to memorize which is which.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 10:43 pm
by Chem_Mod
Javier_Ochoa_DIS_3J wrote:
So the main equation you need is Eph = (work function of metal) - Ek (energy of electron ejected)
The above post is not quite correct.
The correct version is: Energy of photon - work function (threshold energy) = Kinetic energy (of ejected electron)
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 10:45 pm
by Raphael_SanAndres3C
Thank you!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 10:55 pm
by 905092269
When will you post the answers?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 10:57 pm
by Chem_Mod
Answers are now posted. Scroll up.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 11:35 pm
by Katie_Duong_1D
Hello!
For question 3, I keep getting 37.2 g NO for my answer, not 31.7g NO. Is this an error in the solutions, or did I do something wrong?
Equation: 4NH3 + 5O2 --> 4NO + 6H2O
(21.1 g NH3/17.04 g/mol NH3) x (4 mol NO/4 mol NH3) (30.01 g/mol NO) = 37.16 g NO, rounded to 37.2 g NO.
Please let me know if something is wrong with my stoichiometry. Thanks for putting this review worksheet together! Garlic bread was good review AND a good meme :)
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sat Nov 03, 2018 11:39 pm
by Katie_Duong_1D
Katie_Duong_3B wrote:Hello!
For question 3, I keep getting 37.2 g NO for my answer, not 31.7g NO. Is this an error in the solutions, or did I do something wrong?
Equation: 4NH3 + 5O2 --> 4NO + 6H2O
(21.1 g NH3/17.04 g/mol NH3) x (4 mol NO/4 mol NH3) (30.01 g/mol NO) = 37.16 g NO, rounded to 37.2 g NO.
Please let me know if something is wrong with my stoichiometry. Thanks for putting this review worksheet together! Garlic bread was good review AND a good meme :)
I just realized that I used the wrong limiting reagent. O2 is the limiting reagent, not NH3. I have 31.7 g NO now.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:30 am
by Chem_Mod
Katie_Duong_3B wrote:
I just realized that I used the wrong limiting reagent. O2 is the limiting reagent, not NH3. I have 31.7 g NO now.
Good! Remember that the amount of product formed is determined by the limiting reactant. Therefore, the reactant that produces
less product is limiting and will be used to determine theoretical yield.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:27 am
by sonalivij
Could someone explain 13c to me?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 9:03 am
by Samantha Chang 2K
sonalivij wrote:Could someone explain 13c to me?
-13c is a Heisenberg Uncertainty Principle problem. The equation for this problem is ΔPΔX=>h/4pi. For this equation we need the mass, the velocity(these two values multipled together are ΔP), and we need ultimately to find ΔX.
-We are given the mass which is 2.8 grams so we have to divide the number by 1000 in order to obtain the SI unit of Kg, and you will get .0028 Kg. This number will be used for our M in ΔP.
-We are also given the speed and in order to find ΔV we have to multiply .34 and 2 together because that is the total uncertainty of the speed, negatively and positively. So our ΔV will be .68 m/s.
-With all of these values that we found we only have one unknown value which is ΔX, so we simply just plug the values into the equation. (.0028 Kg)*(.68 m/s)*(ΔX)>=h/4pi
Using some simple algebra you will get ΔX>=2.8*10^-32 meters. Remember to do significant figures too!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 10:06 am
by MichaelMoreno2G
The review session on Saturday that went over this practice midterm really helped prepare me for the exam, thank you.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 10:49 am
by Sydney Takeda 2I
It was so crowded but I appreciate the practice problems and review session so much!!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 10:58 am
by Elle_Mendelson_2K
Hello, for number 6a I keep getting 9.36X10 ^-46 instead of 9.36*10^-11 will someone explain what I am doing wrong? I have looked at another students work and we did the exact same thing but I got a different answer. Please help!!
Thank you!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 11:30 am
by Chem_Mod
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 11:31 am
by Yvonne Du
Hi can anyone explain 8a? I don't know how to correctly word my answers...
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:11 pm
by DavidEcheverri3J
Daisylookinland4B wrote:For #6, how are you supposed to convert the molar mass of GarBreadium to kg in order to use the de broglie equation?
First you must find the weight of a single atom of GarBreadium -> (3.257g/1mol) x (1mol/6.02x20^23atoms) = 5.24x10^-24 g/atoms. Then you multiply by 1000 (1000g in 1 kg). Lastly you plug that in as your m in the de Broglie equation (lambda = h/mv). Ah-hah!
Hope this helped!
Good luck!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:21 pm
by Kevin Tang 4L
For problem 9b, the electron configuration of Chromium is [Ar]3d5 4s1. I thought that the 4s orbital was filled before the 3d orbital. I know Copper and some other elements also follow this trend. Why do these elements only fill one of the two electrons in the 4s or 5s orbitals?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:55 pm
by ThomascnguyenDis1J
Thank you so much for the review session! It really helped clarify so many topics for me.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:56 pm
by RandallNeeDis3K
He really is too good. THE MVP!!!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:59 pm
by RandallNeeDis3K
@Kevin Tang, This is because Chromium strives to fulfill the half-filled orbital energy, making it more stable. So instead of 3p4 4s2, it is 3p5 4s1. This is (I think) the only exception when it comes to electron configuration. (as well as all elements in the same group.)
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 1:59 pm
by RandallNeeDis3K
@Kevin Tang, This is because Chromium strives to fulfill the half-filled orbital energy, making it more stable. So instead of 3p4 4s2, it is 3p5 4s1. This is (I think) the only exception when it comes to electron configuration. (as well as all elements in the same group.)
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 2:17 pm
by juleschang16
For question 4, the answer is given with only 2 sig figs (the answer is 9.9 mL of 9.9x10^-3 L, even though the question uses 3 sig figs. The question is: What volume of 0.0380 M KMnO4 is needed to prepare 250 mL of 1.50 x 10-3 M KMnO4?
(Hint: In these types of problems, the volume containing solute is diluted with water to the final
volume, in this case 250mL). Would 3 sig figs as my answer be acceptable?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 2:25 pm
by Jayde Felix 4H
Why do we multiply by 7 in number 1?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 2:37 pm
by Chem_Mod
juleschang16 wrote:Would 3 sig figs as my answer be acceptable?
250 mL is only 2 sig figs. The number of sig figs you use should depend on the value that has the least sig figs. Therefore, 2 sig figs is correct .
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 2:40 pm
by Bijan Mehdizadeh 1B
Thank you so much for your time and help, Lyndon!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 2:42 pm
by Bijan Mehdizadeh 1B
For number 5, I keep getting .127 M after setting the problem up like (.211 M)(.150 L) = M2(.250 L). But in my notes, I believe Lyndon got 1.69 x 10^-2. I was wondering if anyone knows what I am doing wrong. Thanks!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 3:43 pm
by Yvonne Du
Bijan Mehdizadeh 1E wrote:For number 5, I keep getting .127 M after setting the problem up like (.211 M)(.150 L) = M2(.250 L). But in my notes, I believe Lyndon got 1.69 x 10^-2. I was wondering if anyone knows what I am doing wrong. Thanks!
Hi!
Since the problem stated that only 20ml was removed to the 2nd flask, you put 0.02 as your L1 instead of 0.150 L. Now you should get the right answer.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 4:01 pm
by Sara Sadrolsadat 1G
How is that for the Lewis structure of N2O (question 12c), N can have more than 3 bonds?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 5:37 pm
by Sydney Takeda 2I
For 8b, why can't you use Ek = hc/λ to find the kinetic energy then proceed to solve for frequency using this value + the work function all over h?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 5:47 pm
by Michael Torres 4I
Sydney Takeda 1G wrote:For 8b, why can't you use Ek = hc/λ to find the kinetic energy then proceed to solve for frequency using this value + the work function all over h?
The variable c incorporates the speed of light into the equation, but ejected electrons cannot travel at the speed of light. Therefore this cannot work. You can only use the speed of light when working with photons.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 6:07 pm
by Chem_Mod
Sara Sadrolsadat 1G wrote:How is that for the Lewis structure of N2O (question 12c), N can have more than 3 bonds?
Nitrogen must have an octet. We will often see Nitrogen with 3 bonds and one lone pair so that it has an octet and it has 0 formal charge, but sometimes it can have 4 bonds and no lone pairs, giving it a +1 formal charge.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 6:09 pm
by Chem_Mod
Sydney Takeda 1G wrote:For 8b, why can't you use Ek = hc/λ to find the kinetic energy then proceed to solve for frequency using this value + the work function all over h?
The formula you have presented is NOT for kinetic energy. That formula is only for energy of photons (light). Since the problem gave only the wavelength of the electron, and since an electron is not a photon, your method will not work.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 6:32 pm
by Hilda Sauceda 3C
Can anyone explain the reasoning for why 6d is true.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 6:43 pm
by juleschang16
Samantha Chang wrote:sonalivij wrote:Could someone explain 13c to me?
-13c is a Heisenberg Uncertainty Principle problem. The equation for this problem is ΔPΔX=>h/4pi. For this equation we need the mass, the velocity(these two values multipled together are ΔP), and we need ultimately to find ΔX.
-We are given the mass which is 2.8 grams so we have to divide the number by 1000 in order to obtain the SI unit of Kg, and you will get .0028 Kg. This number will be used for our M in ΔP.
-We are also given the speed and in order to find ΔV we have to multiply .34 and 2 together because that is the total uncertainty of the speed, negatively and positively. So our ΔV will be .68 m/s.
-With all of these values that we found we only have one unknown value which is ΔX, so we simply just plug the values into the equation. (.0028 Kg)*(.68 m/s)*(ΔX)>=h/4pi
Using some simple algebra you will get ΔX>=2.8*10^-32 meters. Remember to do significant figures too!
In the problem it says that the speed is 373.34 +/- 0.34 m/s. Where are you getting the 2 from?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 6:47 pm
by melissa_dis4K
For 8B I understand that we need to use E(photon) - work function = kinetic energy. We are given the wavelength and work function. However, I don't know where to use the wavelength since E(photon)=h*frequency and we have the given work function and Kinetic energy= 1/2 Mass* Velocity(unknown). Can someone please explain how to solve this? Thank you!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 6:52 pm
by Eruchi Okpara 2E
Thank you so much Lyndon!!!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 6:55 pm
by Anand Narayan 1G
For number 11, part b why would delta E be a negative value? If the problem is asking for the change in energy from n = 6 to n = 4, and the formula is
E = -hR(1/n1^2 - 1/n2^2), the energy released from n = 6 to n = 4 equals 7.5689 x 10^-20 J. So, why is the answer negative?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 7:31 pm
by Chem_Mod
Hilda Sauceda 3C wrote:Can anyone explain the reasoning for why 6d is true.
As discussed at the review session, a particle with a debroglie wavelength larger than 10^-18 exhibits measurable wavelike properties
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 7:33 pm
by Chem_Mod
juleschang16 wrote:In the problem it says that the speed is 373.34 +/- 0.34 m/s. Where are you getting the 2 from?
Uncertainty is the range of values that we are unsure about. This means taking the highest possible speed, then subtracting the lowest possible speed. This will give you the uncertainty. You will find that doing so is the same as multiplying 0.34 by 2.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 7:34 pm
by Chem_Mod
Anand Narayan 1G wrote:For number 11, part b why would delta E be a negative value? If the problem is asking for the change in energy from n = 6 to n = 4, and the formula is
E = -hR(1/n1^2 - 1/n2^2), the energy released from n = 6 to n = 4 equals 7.5689 x 10^-20 J. So, why is the answer negative?
Delta E is the change in energy. When you go from 6 to 4, you are going from a higher energy state to a lower energy state. When going from high to low, the
change is negative. The electron therefore
lost energy. The energy lost takes the form of a photon, and photons always have positive energy.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 7:36 pm
by melissa_dis4K
Anand Narayan 1G wrote:For number 11, part b why would delta E be a negative value? If the problem is asking for the change in energy from n = 6 to n = 4, and the formula is
E = -hR(1/n1^2 - 1/n2^2), the energy released from n = 6 to n = 4 equals 7.5689 x 10^-20 J. So, why is the answer negative?
Remember that the change in energy is that of the final energy (n=4) minus the initial energy (n=6). This should give you a negative change in energy. Hope this helps!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 7:48 pm
by Edward Xie 2E
What determines the energy of a Lewis structure? Formal charges of all the atoms added together?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 8:14 pm
by Michelle Nwufo 2G
Yvonne Du wrote:Bijan Mehdizadeh 1E wrote:For number 5, I keep getting .127 M after setting the problem up like (.211 M)(.150 L) = M2(.250 L). But in my notes, I believe Lyndon got 1.69 x 10^-2. I was wondering if anyone knows what I am doing wrong. Thanks!
Hi!
Since the problem stated that only 20ml was removed to the 2nd flask, you put 0.02 as your L1 instead of 0.150 L. Now you should get the right answer.
I'm confused as to why .02 L would be the initial volume, if when we calculate the initial molarity (mol/L of KMnO4) in the compound we use .150 L, rather than .020L?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 8:15 pm
by Hilda Sauceda 3C
does anyone know how to solve 8b? i am having trouble with it.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 8:27 pm
by Albert_Luu3K
Thank you so much, Lyndon. These are going to help me a lot as I'm last minute cramming.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 8:33 pm
by melissa_dis4K
juleschang16 wrote:Samantha Chang wrote:sonalivij wrote:Could someone explain 13c to me?
-13c is a Heisenberg Uncertainty Principle problem. The equation for this problem is ΔPΔX=>h/4pi. For this equation we need the mass, the velocity(these two values multipled together are ΔP), and we need ultimately to find ΔX.
-We are given the mass which is 2.8 grams so we have to divide the number by 1000 in order to obtain the SI unit of Kg, and you will get .0028 Kg. This number will be used for our M in ΔP.
-We are also given the speed and in order to find ΔV we have to multiply .34 and 2 together because that is the total uncertainty of the speed, negatively and positively. So our ΔV will be .68 m/s.
-With all of these values that we found we only have one unknown value which is ΔX, so we simply just plug the values into the equation. (.0028 Kg)*(.68 m/s)*(ΔX)>=h/4pi
Using some simple algebra you will get ΔX>=2.8*10^-32 meters. Remember to do significant figures too!
In the problem it says that the speed is 373.34 +/- 0.34 m/s. Where are you getting the 2 from?
You have to take the +/- 0.34 m/s and multiply by two to get the change in velocity. For example, if the velocity is (100 m/s +/- 1 m/s) then delta velocity is 2 m/s. That is, we took 1 * 2. You can also see it as 99-101 m/s which is also a delta velocity of 2 m/s. Hope this helps!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 8:39 pm
by Olivia L 4E
For question 12c, why is it better to have a triple bond with the two nitrogen atoms instead of double bonds on nitrogen and oxygen? The charge is both the same.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 8:54 pm
by chrisdinkel_4E
For question 13A, can someone please explain why the answer is 2.681x10^-26? I got the 2.681, but for some reason I got x10^-23.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 8:59 pm
by chrisdinkel_4E
Olivia L 3D wrote:For question 12c, why is it better to have a triple bond with the two nitrogen atoms instead of double bonds on nitrogen and oxygen? The charge is both the same.
It is better to have a triple bond as opposed to a double bond because Oxygen is the more electronegative atom.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 9:04 pm
by nataliefgarcia3I
Thank you so much this was very helpful!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 9:06 pm
by Hilda Sauceda 3C
Thank you for this review it has really been helping me understand things better!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 9:09 pm
by Kirsty Star 2H
I don't understand why in order of increasing ionization energy, it goes C O N F. I thought it would be C N O F. Anyone know why?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 9:30 pm
by Edward Xie 2E
I don't understand why in order of increasing ionization energy, it goes C O N F. I thought it would be C N O F. Anyone know why?
Nitrogen is an exception (so is phosphorus) to the trend in that it has a half-filled subshell, 2p^3, which makes it harder for an electron to be removed.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 9:32 pm
by Edward Xie 2E
For question 13A, can someone please explain why the answer is 2.681x10^-26? I got the 2.681, but for some reason I got x10^-23.
Did you remember to convert the mass into kg?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 9:57 pm
by Yiting_Gong_4L
Thank you for these practice problems!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 10:19 pm
by Lina Petrossian 1D
How do you go about solving the first problem?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 10:39 pm
by StudentD2B
I tried the first problem and got it very very wrong. How would I solve it correctly?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 10:46 pm
by StudentD2B
Lina Petrossian 3D wrote:How do you go about solving the first problem?
https://wps.prenhall.com/wps/media/obje ... h0305.htmlSee for exact solution
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 10:55 pm
by Josceline 3J
Thank you so much this is really helpful!!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 11:13 pm
by 404817859
can someone further explain number 6c? I'm not sure how the wavelengths correspond with lower frequency
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 11:30 pm
by 504999383
I wasn't able to make it to the review session, can someone who did go post their notes? It'd be super helpful!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 11:33 pm
by 404817859
9c) I know the answer is sodium but why don't we put the positive 1 charge :)
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 11:41 pm
by Natalie Liu 4I
Thank you for the extra practice!
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Sun Nov 04, 2018 11:55 pm
by Imelda Mena 3I
Question 6b is asking if a Helium atom or a "new" element GarBreadium have longer wavelengths. The answer key says the GarBreadium will have a longer wavelength but isn't it He because it is less heavy, therefore the denominator in de Broglie's equation
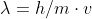
be smaller, corresponding to a bigger wavelength ? Is this a typo ?
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Mon Nov 05, 2018 12:19 am
by StudentD2B
Could someone explain 8b to me. Please tell me where I went wrong here. I found:
wavelength: 1.10 * 10^-9m
P=7.29 * 10^-43
V=8.00 * 10^-13
Work Function= 2.50 * 10^-19 J/atom
Kinetic Energy= 7.00 * 10^-55
Final Frequency= 5.94 * 10^-10
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Mon Nov 05, 2018 12:23 am
by StudentD2B
Jk final frequency I get 3.77 * 10^14. Why is this off
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Mon Nov 05, 2018 12:34 am
by Kevin Tang 4L
304981930 wrote:Jk final frequency I get 3.77 * 10^14. Why is this off
This is because you didn't add the KE with the threshold value. You only did 2.5x^-19 =hv
v=3.8x10^14
If you add the KE, you should get the answer 6.8*10^14 Hz
KE= 1/2mv^2
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Mon Nov 05, 2018 12:36 am
by Kevin Tang 4L
404817859 wrote:can someone further explain number 6c? I'm not sure how the wavelengths correspond with lower frequency
Refer to the equation c=(lambda)(v), you can rewrite this as c/(lambda)=v or c/v=(lambda). You can see they are inversely related; when wavelength increases, the frequency decrease and vice versa.
Re: MIDTERM PRACTICE - Garlic Bread Review Session
Posted: Mon Nov 05, 2018 12:37 am
by Kevin Tang 4L
Imelda Mena 3I wrote:Question 6b is asking if a Helium atom or a "new" element GarBreadium have longer wavelengths. The answer key says the GarBreadium will have a longer wavelength but isn't it He because it is less heavy, therefore the denominator in de Broglie's equation
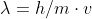
be smaller, corresponding to a bigger wavelength ? Is this a typo ?
Helium actually weights more because it is roughly 4g/mol whereas GarBreadium is 3.157g/mol. I made this mistake too, (you have to add the neutrons and protons) 2+2=4