Page 1 of 2
FINAL PRACTICE - Lyndon's Churro Review Session [ENDORSED]
Posted: Sun Dec 02, 2018 5:35 pm
by Chem_Mod
The title is called "Churro" so that you can search "Churro" and easily find this post!
This is your favorite UA, Lyndon Bui, and I have created many practice problems for your final. The questions are modeled after previous test and exam questions, but it may not have everything you need to know. This covers only material after the midterm. I already covered pre-midterm material in a midterm review session.
Please complete these problems before my review session. My review session this quarter (Fall18) will be Friday, Dec 7, 4-6:50pm, in Haines 39.
I will go over each problem in detail during my review session. The answers, but not full solutions or work, will be posted here at this post after the review session. Any errors in the test will be posted below as well. If you have questions, please reply to this post and I will answer them if another student does not.
Printed copies will NOT be provided at the review session.
Link to Download Problems: Problems no longer available. Quarter has ended. See Course website and syllabus for most up-to-date material.
***This is not indicative of the structure, length, or format of the actual final. Treat these as extra practice problems. ***
Errors: #34 should say "1.0L Flask instead of 1L Flask"
Link for Answers: No Longer Available
Happy Studying and Good Luck!
-Lyndon Bui, UA
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sun Dec 02, 2018 7:53 pm
by Phil Timoteo 1K
Thank you so much!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sun Dec 02, 2018 9:04 pm
by Sydney Takeda 2I
bless your heart for these practice problems and review sessions
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sun Dec 02, 2018 10:17 pm
by Luis_Yepez_1F
Thank you so much for providing us with study material :D
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sun Dec 02, 2018 10:47 pm
by ElliotPourdavoud 1A
Thank you very much for this!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sun Dec 02, 2018 11:49 pm
by yaosamantha4F
Thank you so much for these problems!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Mon Dec 03, 2018 3:49 pm
by michellebui_3L
Best SEA Admit brother ever!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Tue Dec 04, 2018 11:56 pm
by Karla_Ocampo 4E
Thank you very much!!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 2:22 pm
by Jasmine Reddy DIS 1E
Thank you so much for all that you do!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 2:38 pm
by Leela_Mohan3L
Thank you! Does the compound in number 31 have a net charge?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 3:20 pm
by Kevin Tang 4L
Leela_Mohan3L wrote:Thank you! Does the compound in number 31 have a net charge?
[Ni (NH3)5 NO2]^+2
Because the Nickel has a 3+ charge and the NO2 has a 1- charge, it brings it to a net charge of 2+.
NH3 has no charge.
I hope this helps.
Please correct me if I made a mistake. Thanks
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 3:43 pm
by Leela_Mohan3L
Kevin Tang 4E wrote:Leela_Mohan3L wrote:Thank you! Does the compound in number 31 have a net charge?
[Ni (NH3)5 NO2]^+2
Because the Nickel has a 3+ charge and the NO2 has a 1- charge, it brings it to a net charge of 2+.
NH3 has no charge.
I hope this helps.
Please correct me if I made a mistake. Thanks
That's what I was thinking as well. So then the compound name would have to have "ion" at the end, right?
Thanks!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 4:50 pm
by sallina_yehdego 2E
THANK YOU!!!!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 5:37 pm
by Kenan Kherallah 2C
Hey guys can someone help me with question 21, I really dont know how to approach this since I just get a formal charge of +2 in total for the ligands which doesnt seem right
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 8:34 pm
by Avery Zuelch 1D
Thank you!! Your sessions and review problems are always so helpful!!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 8:41 pm
by Chem_Mod
Kenan Kherallah 1A wrote:Hey guys can someone help me with question 21, I really dont know how to approach this since I just get a formal charge of +2 in total for the ligands which doesnt seem right
I will go over this in detail on Friday! -Lyndon
But for now.. think about how you would determine the oxidation state of a transition metal for a simpler complex then apply the same concepts to this larger molecule.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 9:04 pm
by Vana Mirzakhani 3I
Thank you so much for having taken the time to create a review packet for us Lyndon! Appreciate it!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 9:11 pm
by Daniel Bowen 3I
For question 34, once I have the Molarity of both compounds how do I find the pH with the two Molarities of HCl and CaO?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 9:51 pm
by kamalkolluri
Daniel Bowen 3I wrote:For question 34, once I have the Molarity of both compounds how do I find the pH with the two Molarities of HCl and CaO?
I'm not completely sure if this is right but, for #34, I did the neutralization rxn between HCl and CaO. One of them is limiting, so if you use all of it up, you form water. The excess that's left over you can use to find the pH.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 10:00 pm
by Kirsty Star 2H
How do we know the shape of a coordination compound? Does it have to do with the number of bonded ligands?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 10:06 pm
by Elena Maneffa 1E
Thank you so much for all your help. This is extremely appreciated.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Wed Dec 05, 2018 11:47 pm
by 504999383
Thanks Lyndon you're a real one!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 1:38 am
by josephyim1L
how might one solve question 34? Should the overall concentration be found or is it only necessary to find the concentration of HCL is 1 Liter of water?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 8:11 am
by Ashley Odibo Dis3E
Thank you! This is really appreciated.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 2:14 pm
by Chem_Mod
josephyim1H wrote:how might one solve question 34? Should the overall concentration be found or is it only necessary to find the concentration of HCL is 1 Liter of water?
I will go over this in detail on Friday (tomorrow) -Lyndon
As a hint, remember that you only look at Hydronium ions and hydroxide ions when determining pH or pOH. Acids generate hydronium ions while bases generate hydroxide ions. The two neutralize each other.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 2:17 pm
by Angela Cong 3C
Thank you so much to my favorite UA. I greatly appreciate your practice problems!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 3:17 pm
by Carolina Lechuga
These practice worksheets are my fave !! Everyone I know is going to your review session! You really clear things up.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 4:03 pm
by Carolina Lechuga
Can anyone help me with the bond angle for number one ?? I have difficulty determining bond angles so for this problem what would it be and why?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 5:09 pm
by Kevin Tang 4L
Carolina Lechuga wrote:Can anyone help me with the bond angle for number one ?? I have difficulty determining bond angles so for this problem what would it be and why?
This angle will be slightly less than 120 degrees. This is because it is sp2 hybridized, and has a VSEPR formula of AX2E the shape is bent. The lone pair repels the oxygens more than a bonding pair, making the angle less than 120 degrees.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 5:36 pm
by Kunseo Yook 2E
Thank you for the problems!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Thu Dec 06, 2018 11:04 pm
by 1K Kevin
The real mvp here
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 12:14 pm
by Dayna Pham 1I
thanks :P
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 12:38 pm
by Jayde Felix 4H
Thanks Lyndon you're the best
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 2:00 pm
by MichaelMoreno2G
Thank you for this! The one for the midterm really helped out and I hope this one does too. What will be the final topic covered during the review session?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 2:02 pm
by ariellasarkissian_3H
Thank you so much!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 3:15 pm
by Elle_Mendelson_2K
Thank you Lyndon! Is this the same level of difficulty as the final will be?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 3:15 pm
by Elle_Mendelson_2K
Will someone please explain number 20?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 3:41 pm
by Sydni Stewart
You are a chemistry saint. Thank you.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 3:42 pm
by Elle_Mendelson_2K
Will someone please explain 22
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 5:07 pm
by Karla_Ocampo 4E
Hey guys! Im at another review for another final *crying emoji* , can someone PLEASE send me pictures of their notes?? I would greatly appreciate it!! my email is
itzkar1@g.ucla.edu
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 5:11 pm
by AustinGrove3B
Elle_Mendelson_4I wrote:Will someone please explain 22
Myoglobin has one heme group, which is what that Fe N structure is. Each heme group can hold one oxygen, therefore myoglobin holds 1 oxygen. Hemoglobin has 4 heme groups.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 5:13 pm
by AustinGrove3B
Elle_Mendelson_4I wrote:Will someone please explain number 20?
Iron actually likes to have 6 bonds... coordination number 6. This Fe in the structure is bound to 4 nitrogens, but will also bind to a histine group and a structure that binds to oxygen, creating 6 bonds. 6 bonds creates an octahedral structure.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 6:44 pm
by Daniel Bowen 3I
Can someone please explain 33 part C?
33c
Posted: Fri Dec 07, 2018 6:47 pm
by Meachelle_Lum_1I
For 33c, since we have a weak base, we will have less OH since it doesn’t completely dissociate. However, since less OH means more H3O, this means less pOH should mean more pH. Why did Lyndon say lower pH instead?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 7:18 pm
by Daniel Bowen 3I
Will someone please explain question 40 part B and C
Re: 33c
Posted: Fri Dec 07, 2018 7:58 pm
by Lina Petrossian 1D
Meachelle_Lum_3C wrote:For 33c, since we have a weak base, we will have less OH since it doesn’t completely dissociate. However, since less OH means more H3O, this means less pOH should mean more pH. Why did Lyndon say lower pH instead?
More H30 means more proton concentration, meaning a lower (more acidic) pH value.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 7:58 pm
by Lina Petrossian 1D
When will answers be posted?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 8:06 pm
by MichelleRamirez_2F
Can someone explain why hemogrlobin is tetradentrate but cisplatin is monodentrate?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 8:48 pm
by Ryan Danis 1J
On question 38, how come the LDF’s are the determining factor instead of dipole-dipole in determining the boiling point?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 8:49 pm
by Shivangi_2J
Michelle Ramirez_4A wrote:Can someone explain why hemogrlobin is tetradentrate but cisplatin is monodentrate?
hemoglobin and cisplatin are not ligands, so they aren't any kind of "dentate." We only describe ligands as being bidentate, tridentate, etc.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 9:11 pm
by KatelinTanjuaquio 1L
Ryan Danis 1K wrote:On question 38, how come the LDF’s are the determining factor instead of dipole-dipole in determining the boiling point?
H2Se has stronger London dispersion forces, while H2S has stronger dipole-dipole forces. However, because we are given that H2Se has a higher boiling point, all we know that is that the london dispersion forces were stronger and thus dominated.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 9:14 pm
by KatelinTanjuaquio 1L
Daniel Bowen 3I wrote:Will someone please explain question 40 part B and C
For part B:
We would expect ozone to have two bond lengths are the same. This is because it is a resonance structure. In resonance structures, the bond lengths are the same--the average of its bond lengths.
For part C:
O2 would have the strongest bond because it has a double bond and thus is shorter.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 9:43 pm
by Nicolle Fernandez 1E
Thanks! When are the answers going to be posted?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 10:15 pm
by Leslie Cheng 4B
On question 21, can someone explain why Fe has an oxidation state of +2? I get that this is because of the nitrogens, but how do the nitrogens come to have a charge of -2? I was trying to calculate the formal charge but kept getting a positive charge on the nitrogens, not a negative charge.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 11:06 pm
by Joussie Camacho 4I
Leslie Cheng 4B wrote:On question 21, can someone explain why Fe has an oxidation state of +2? I get that this is because of the nitrogens, but how do the nitrogens come to have a charge of -2? I was trying to calculate the formal charge but kept getting a positive charge on the nitrogens, not a negative charge.
This is because we are told that the overall charge of the molecule is neutral. So if the nitrogens all have a charge of -2, then Fe has to have a charge of +2 in order for the overall charge to be 0. In order to find the charge of the nitrogen ligand, you have to look at each nitrogen when it is not bound to Fe (so you replace each n-Fe bond with a lone pair) and also fill in the rest of the lone pair necessary for each nitrogen to have a full octet (again, while unbound). Once you do this, you can find the formal charge of each nitrogen. There are 4 nitrogens, 2 of them had two pairs of lone pair electrons and 2 single bonds, so those had a formal charge of -1 (5-6 = -1) each, and the other two nitrogens had one pair of lone electrons and one single bond and one double bond, so those had a formal charge of 0 (5-5 = 0). The whole ring around Fe is 1 single ligand because it's all connected, so if we add all of the nitrogen charges together, the charge of the ligand is -2.
This is really hard to explain without the picture but I hope it helped somehow!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Fri Dec 07, 2018 11:51 pm
by Destiny Diaz 4D
Thank you for your help ! When will the answers be posted ? :)
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 12:11 am
by CaminaB_1D
can someone explain 23 and why porphyrin ligand is tetradentrate
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 12:35 am
by isarose0
CaminaB_1D wrote:can someone explain 23 and why porphyrin ligand is tetradentrate
Porphyrin is tetradentate because there are four binding sites of the ligand (the four N's in the diagram) to the Fe. The prefix to the dentate has to do with how many binding sites there are for a single ligand.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 1:38 am
by Chem_Mod
Answers are posted now at the top. Scroll up:)
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 2:30 am
by Richard Ku 4H
Thank you so much for this!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 10:23 am
by Reese - Dis 1G
Can someone explain 27? I thought you only added -ate when the compound was negative.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 12:10 pm
by ariellasarkissian_3H
Can someone explain the bond angles in number 18?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 12:19 pm
by ariellasarkissian_3H
How do we determine the hybridization on the lone pair? For example, if O is bonded to two atoms and has two lone pairs, wouldn't it just be sp3 or is there another specific notation for the lone pair I am missing?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 1:08 pm
by yaosamantha4F
Thank you for posting these!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 1:57 pm
by Esther Lee 4H
thank you!!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 2:06 pm
by Chris Freking 2G
Reese - Dis 1G wrote:Can someone explain 27? I thought you only added -ate when the compound was negative.
If K
2 has a +2 charge, then [Ni(CN)
4] must have a -2 charge since the overall compound is neutral. It's called "nickelate" because the compound in the brackets has the -2 charge.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 2:16 pm
by Venya Vaddi 1L
ariellasarkissian_3H wrote:How do we determine the hybridization on the lone pair? For example, if O is bonded to two atoms and has two lone pairs, wouldn't it just be sp3 or is there another specific notation for the lone pair I am missing?
In the same way that you do when determining shape, count the regions of electron density. If O has two lone pairs and two bonding pairs, the total number of regions of electron density is 4, so the hybridization is sp3. There are 4 sp3 orbitals formed: two of them hold one lone pair each, and the remaining two are used in the bonding pairs.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 2:47 pm
by Kelsey Li 3B
thank you for the final review it really helped!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 3:25 pm
by BenJohnson1H
So when calculating the pH of an acid and base reaction, would you need to put both into one equation or write equations separately for each?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 3:39 pm
by 904837647
THANK YOU SO MUCH!!! THIS IS DEFINITELY A HUGE HELP!!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 3:42 pm
by 904837647
Will the final be similar to this practice final?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 3:54 pm
by Sydney Takeda 2I
For #31, would it also be correct to put NO2 in the complex or does it have to be ONO?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 3:57 pm
by Celine Hoh 2L
Thank you! Does anyone know which other TAs or UAs post worksheets as well (besides Lyndon and Karen)?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:07 pm
by Ahmet_Dikyurt_3L
Can someone explain 33b?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:12 pm
by Simran Rai 4E
These practice problems and review sessions are so helpful. Thank you Lyndon!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:18 pm
by Imelda Mena 3I
ariellasarkissian_3H wrote:Can someone explain the bond angles in number 18?
For number 18, you have to look at the structure with all bonds, atoms, and lone pairs to make it easy to see what is going on. Once you have a structure that includes every thing see what shapes you have for each atom and from the shape determine what the different bond angles would be. For example, the less than 109.5 degrees comes from the bent shape of the Oxygen with the two lone pairs and two Carbon atoms attached to it. The 109.5 degrees angle comes from the tetrahedral shape of the CH3 at the far right of the molecule. The 120 degrees can be seen in any of the trigonal planar shapes, and lastly the 180 degrees is from the CN bond that is composed of a triple bond and is therefore linear.
Hope this helps!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:21 pm
by Imelda Mena 3I
Sydney Takeda 1G wrote:For #31, would it also be correct to put NO2 in the complex or does it have to be ONO?
I would say it has to be ONO, nitrito, because if you write NO2 that can be mistaken with nitro. Which seem similar but are not because the central atom for each differentiates.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:26 pm
by Imelda Mena 3I
Ahmet_Dikyurt_3L wrote:Can someone explain 33b?
For this question it's crucial to realize that NaOH is a strong base and therefore we know that it completely dissociates. Once we have identified this, we know that 0.55M NaOH will produce 0.55 OH- because it completely dissociates. From here all that is left is plug in the concentration of the OH- into the pOH formula,
)
, and then once you get the pOH which in this problem is = 0.2596 you use this to get pH by using,
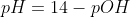
and get a pH of 13.740.
For this problem also remember that the significant figure rules are different.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:30 pm
by Simran Athwal-Dis 3A
Thank you so much, this was so helpful.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:34 pm
by 905085650
Thank you for your help this quarter!!!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:35 pm
by Lisa Werner 2F
thank you so much!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:52 pm
by bonnie_schmitz_1F
For question 18, I'm having trouble finding the 180˚ bond. Which bond would it be?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 4:55 pm
by Krista Mercado 1B
Thank you so much for the final review and practice problems!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 5:01 pm
by Kathryn 1F
Imelda Mena 3I wrote:Sydney Takeda 1G wrote:For #31, would it also be correct to put NO2 in the complex or does it have to be ONO?
I would say it has to be ONO, nitrito, because if you write NO2 that can be mistaken with nitro. Which seem similar but are not because the central atom for each differentiates.
I think we should specify ONO-, but doesn't both NO2- and ONO- had the same lewis structure (with N as central), but the difference was what atom was bound to the metal, with N binding for NO2- and O binding for ONO-? I got this looking at the "naming coordination compounds" page on lavelle's website
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 5:16 pm
by LeannaPhan14BDis1D
this was really helpful and so was the review session thank you!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 5:20 pm
by yuetao4k
Thank you for the churro review! I also really liked garlic bread(:
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 5:27 pm
by Kathryn 1F
Can someone explain 41d - how many atoms in thymine could form a hydrogen bond. The answer is 4 but I only see 2--on either oxygen. Where are the other 2?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 6:26 pm
by Imelda Mena 3I
Kathryn 3D wrote:Imelda Mena 3I wrote:Sydney Takeda 1G wrote:For #31, would it also be correct to put NO2 in the complex or does it have to be ONO?
I would say it has to be ONO, nitrito, because if you write NO2 that can be mistaken with nitro. Which seem similar but are not because the central atom for each differentiates.
I think we should specify ONO-, but doesn't both NO2- and ONO- had the same lewis structure (with N as central), but the difference was what atom was bound to the metal, with N binding for NO2- and O binding for ONO-? I got this looking at the "naming coordination compounds" page on lavelle's website
Yes, you are right! I messed up, I did NOT mean to say that the Lewis structure was different sorry for the confusion! The difference is the atom to which the metal will bind to.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 6:27 pm
by Imelda Mena 3I
Kathryn 3D wrote:Can someone explain 41d - how many atoms in thymine could form a hydrogen bond. The answer is 4 but I only see 2--on either oxygen. Where are the other 2?
The other two come from the two Nitrogens in the structure.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 6:33 pm
by Daniela Alvarado 3B
for #24, why can't we find the oxidation state of Fe by finding the nitrogen' formal charges while attached?
Thanks in advance!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 6:42 pm
by gabbym
Thank you for taking the time to make this for us!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 6:54 pm
by Michael Nirula
Can someone explain 21 aka why is the oxidation state in poryphorin +2?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 6:57 pm
by Kaylee Kang 1G
Thank you SO MUCH!!
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 7:08 pm
by Alan Chang
Which HW question is question 17 similar to?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 7:21 pm
by marg44
Can someone explain 41c? I'm confused as to why the nitrogen with the lone pair can accept the proton but not the oxygen.
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 7:44 pm
by bonnie_schmitz_1F
I think I understand the basis of how to do number 34, but I keep getting a pH of 2.10. Did anyone else make the error same/know what my error is?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 7:58 pm
by Madelyn Cearlock
Can someone please explain why in number 21 the oxidation number for iron is 2+?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 8:27 pm
by Madelyn Cearlock
Can someone please explain number 28?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 8:33 pm
by Ashley Odibo Dis3E
What is a porphyrin ligand and will we be asked about ligands like this on the test?
Re: FINAL PRACTICE - Lyndon's Churro Review Session
Posted: Sat Dec 08, 2018 8:47 pm
by Ashley Odibo Dis3E
Joussie Camacho 4I wrote:Leslie Cheng 4B wrote:On question 21, can someone explain why Fe has an oxidation state of +2? I get that this is because of the nitrogens, but how do the nitrogens come to have a charge of -2? I was trying to calculate the formal charge but kept getting a positive charge on the nitrogens, not a negative charge.
This is because we are told that the overall charge of the molecule is neutral. So if the nitrogens all have a charge of -2, then Fe has to have a charge of +2 in order for the overall charge to be 0. In order to find the charge of the nitrogen ligand, you have to look at each nitrogen when it is not bound to Fe (so you replace each n-Fe bond with a lone pair) and also fill in the rest of the lone pair necessary for each nitrogen to have a full octet (again, while unbound). Once you do this, you can find the formal charge of each nitrogen. There are 4 nitrogens, 2 of them had two pairs of lone pair electrons and 2 single bonds, so those had a formal charge of -1 (5-6 = -1) each, and the other two nitrogens had one pair of lone electrons and one single bond and one double bond, so those had a formal charge of 0 (5-5 = 0). The whole ring around Fe is 1 single ligand because it's all connected, so if we add all of the nitrogen charges together, the charge of the ligand is -2.
This is really hard to explain without the picture but I hope it helped somehow!
Thank's for your explanation, I was stuck on this for a while before