For the example we went over for the photoelectric effect lecture:
-If 3.61x10^-19J is required to remove an electron with 0 kinetic energy from a metal surface, what would be the longest wavelength of light that could do this? The answer is 551x10^-9m or 551nm. I was confused by this because I was under the impression that the wavelength has to be in the ultraviolet "zone" in order to have enough energy to eject an electron. But UV wavelengths are between 100-400nm. Does ultraviolet light vs. visible light not matter when looking at having enough energy to eject an electron?
Photoelectric Effect Lecture Question
Moderators: Chem_Mod, Chem_Admin
-
Rochelle Ellison 2H
- Posts: 29
- Joined: Wed Sep 21, 2016 3:00 pm
Re: Photoelectric Effect Lecture Question
The energy needed to eject an electron depends on the metal and its work function or threshold energy. For example the threshold energy for sodium metal is around 2.3 eV (1 eV = 1.60 X 10^-19). So approximately E threshold = 3.68 X 10^-19 J. Using the formula λ = (hc)/E you find that the wavelength is around 540 nm which corresponds to green light. So yes visible light has enough energy to eject electrons from metals that have lower threshold energies like sodium metal.
-
Kimberly_Hoh_2A
- Posts: 20
- Joined: Wed Sep 21, 2016 3:00 pm
Re: Photoelectric Effect Lecture Question
I also have a question that relates to the exapmle we did in class. For that problem we were given the equation 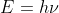
and in this case E was the threshold energy (3.61x10-19) which is the same as the energy of the photon because the electron's kinetic energy was zero. My question is should E in this equation always be the energy of the photon? Or could it also be the threshold energy if the energy of the photon is not given, or even the kinetic energy of the electron? I feel like I am overthinking this and I apologize if this question is silly or doesn't make sense. I'm also sorry if this is in the wrong thread.
and in this case E was the threshold energy (3.61x10-19) which is the same as the energy of the photon because the electron's kinetic energy was zero. My question is should E in this equation always be the energy of the photon? Or could it also be the threshold energy if the energy of the photon is not given, or even the kinetic energy of the electron? I feel like I am overthinking this and I apologize if this question is silly or doesn't make sense. I'm also sorry if this is in the wrong thread.
-
Nathan Mallipeddi 2H
- Posts: 23
- Joined: Fri Jul 22, 2016 3:00 am
Re: Photoelectric Effect Lecture Question
The E in the equation E=hv is always the energy of the photon. The only time E is the threshold energy is when you are told in the problem that the kinetic energy is 0 because then the energy of a photon and threshold energy must be equal. I think if you are not given the energy of a photon, assume that E is the energy of a photon and not the threshold energy unless you are specifically told that kinetic energy is 0. Otherwise it is just the energy of the photon!
-
Kimberly_Hoh_2A
- Posts: 20
- Joined: Wed Sep 21, 2016 3:00 pm
Return to “Photoelectric Effect”
Who is online
Users browsing this forum: No registered users and 6 guests

