1.9
Moderators: Chem_Mod, Chem_Admin
-
Alysia Garcia 1B
- Posts: 40
- Joined: Wed Feb 21, 2018 3:02 am
1.9
For this problem, you are given the frequency 8.7x10^14 HZ. I understand that you have to divide that by the speed of light, 3.00x10^8 ms-1, to find the wavelength but how would you convert that to nm units?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: 1.9
First off, to find the wavelength, you actually divide the speed of light by frequency from the equation: 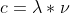 . After finding wavelength, your answer should be in meters, m. To convert to nm, you should know that there are
. After finding wavelength, your answer should be in meters, m. To convert to nm, you should know that there are 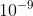 m in one nm.
m in one nm.
-
Jocelyn Fermin1J
- Posts: 49
- Joined: Tue Nov 14, 2017 3:01 am
-
MadelynNguyen1F
- Posts: 59
- Joined: Fri Apr 06, 2018 11:04 am
-
Jared Pagal 1J
- Posts: 24
- Joined: Fri Apr 06, 2018 11:04 am
Re: 1.9
The second value in the table is given as Energy equals 3.3x10^-19 J. I divided by Planck's constant using the equation E=hv and the result was 4.980x10^14. Is it unusual to have a wavelength that long?
-
Beverly Shih 1K
- Posts: 34
- Joined: Wed Nov 15, 2017 3:01 am
- Been upvoted: 1 time
Re: 1.9
I think what you calculated was actually frequency, not wavelength. In the equation E = hv, v stands for frequency so you would want to use E = hc/lambda and solve for lambda instead.
A wavelength of 4.980x10^14 Hz seems more than reasonable. For reference, red light has a frequency of about 4.3x10^14 Hz.
A wavelength of 4.980x10^14 Hz seems more than reasonable. For reference, red light has a frequency of about 4.3x10^14 Hz.
-
Gisselle Sainz 2F
- Posts: 76
- Joined: Wed Feb 21, 2018 3:00 am
Return to “Photoelectric Effect”
Who is online
Users browsing this forum: No registered users and 2 guests

