Photoelectric Effect Equation
Moderators: Chem_Mod, Chem_Admin
-
Kimberly Bauer 4E
- Posts: 51
- Joined: Thu Jul 25, 2019 12:17 am
Photoelectric Effect Equation
Is there an equation we need to use with the photoelectric effect and if there is what do we use and when do we know when to use it (keywords etc.)?
-
Sanjana Munagala_1j
- Posts: 103
- Joined: Sat Aug 24, 2019 12:17 am
- Been upvoted: 1 time
Re: Photoelectric Effect Equation
The equation to use is:
E(photon)= work function(or threshold energy) + Kinetic energy of electron
Honestly I would write down this equation whenever you have a question involving electrons being ejected from a metal.
Hope that helps!
E(photon)= work function(or threshold energy) + Kinetic energy of electron
Honestly I would write down this equation whenever you have a question involving electrons being ejected from a metal.
Hope that helps!
-
Isabel Day 1D
- Posts: 48
- Joined: Fri Aug 09, 2019 12:15 am
Re: Photoelectric Effect Equation
There's 2 equations we use for the photoelectric effect.
E= hv (where E: photon energy, h: Planck's constant= 6.63x10^-34, and v= frequency)
This equation can also be written as E= hc/wavelength (where c: speed of light= 3.00x10^8)
We can use this equation if we're given (1) the energy of the photon of light shined on the metal in the photoelectric effect or (2) the frequency/wavelength of the photons
Ek (kinetic energy)= 1/2mV^2 (where m= mass of the emitted electron and V= velocity of the emitted electron)
We can use this equation to find the excess energy of the emitted electron. Ek= E(photon)- E(remove e)= E(excess)
E= hv (where E: photon energy, h: Planck's constant= 6.63x10^-34, and v= frequency)
This equation can also be written as E= hc/wavelength (where c: speed of light= 3.00x10^8)
We can use this equation if we're given (1) the energy of the photon of light shined on the metal in the photoelectric effect or (2) the frequency/wavelength of the photons
Ek (kinetic energy)= 1/2mV^2 (where m= mass of the emitted electron and V= velocity of the emitted electron)
We can use this equation to find the excess energy of the emitted electron. Ek= E(photon)- E(remove e)= E(excess)
-
zoedfinch1K
- Posts: 57
- Joined: Thu Jul 25, 2019 12:16 am
Re: Photoelectric Effect Equation
For analyzing the photoelectric effect, questions can ask you about the kinetic energy of an emitted electron or the wavelength needed to eject the electron. For these problems, you would use the equation for the kinetic energy of the electron Ek = 1/2*me*v2 = 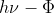 and
and 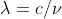 .
.
Return to “Photoelectric Effect”
Who is online
Users browsing this forum: No registered users and 9 guests

