"1B.15 The velocity of an electron that is emitted from a metallic surface by a photon is 3.6 x10^3 km/s . (a) What is the wavelength of the ejected electron? (b) No electrons are emitted from the surface of the metal until the frequency of the radiation reaches 2.50 x10^16 Hz. How much energy is required to remove the electron from the metal surface? (c) What is the wavelength of the radiation that caused pho- toejection of the electron? (d) What kind of electromagnetic radiation was used?"
I was able to get part a, but I couldn't seem to get the correct answers for the other parts. I don't think I am understanding the problem correctly. Can anyone explain the steps for b and c please?
Textbook Problem 1B.15
Moderators: Chem_Mod, Chem_Admin
-
Arieanne De Guzman 2J
- Posts: 55
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 3 times
-
StephanieIb
- Posts: 107
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Textbook Problem 1B.15
For b, you need to use hv=work function + 0(for KE). Then, multiply h by frequency to get your answer (1.66x10^-17 J). For C, use E= work function + 1/2mv^2. Add your work function (1.66x10^-17) and 1/2(9.109x10^-31kg)(3.6x10^6 m/sec)^2. Yous should get 2.25x10^-17 J as your E(pho). Then, using E=hV, divide energy(2.25x10^-17) by h to get the frequency. You should get 3.3957 x10^16 Hz. Then to get wavelength, use wavelength=C/V. You should get 8.8x10^-9m.
-
Annie Tong 2G
- Posts: 51
- Joined: Wed Sep 30, 2020 9:42 pm
- Been upvoted: 1 time
Re: Textbook Problem 1B.15
b) Use the equation hv = work function + 0 (KE). Substitute in v = 2.50 x 10^16 Hz to get work function = 1.66 x 10^-17.
c) Use the equation E = 1/2mv^2 + work function. Use the answer from part b) to substitute work function and v (velocity) = 3.6 x 10^6 m/s (make sure to convert km to m!) and m = electron's mass = 9.109 x 10^-31 kg. After you get E, use the equation wavelength = hc/E to find the wavelength.
Hope this helped!
c) Use the equation E = 1/2mv^2 + work function. Use the answer from part b) to substitute work function and v (velocity) = 3.6 x 10^6 m/s (make sure to convert km to m!) and m = electron's mass = 9.109 x 10^-31 kg. After you get E, use the equation wavelength = hc/E to find the wavelength.
Hope this helped!
-
Halle Villalobos 3E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:52 pm
Re: Textbook Problem 1B.15
Hi! For part b, you would use 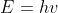 and plug in the given values to get
and plug in the given values to get (2.50*10^{-16}s^{-1})=1.66*10^{-17}J) . For part c, the photon must have enough energy to eject the electron and cause it to move at
. For part c, the photon must have enough energy to eject the electron and cause it to move at 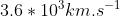 . To find the energy of the photon, add the value found in part a to its kinetic energy
. To find the energy of the photon, add the value found in part a to its kinetic energy ) , which will give you
, which will give you 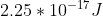 . Remember to convert the velocity from km/s to m/s! To find the wavelength, use the equation
. Remember to convert the velocity from km/s to m/s! To find the wavelength, use the equation 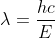 to get 8.8nm. For part d, we know it is in the x-ray/gamma ray region based off the value found in part c. I hope this helps!
to get 8.8nm. For part d, we know it is in the x-ray/gamma ray region based off the value found in part c. I hope this helps!
-
Brandon Carris
- Posts: 49
- Joined: Wed Sep 30, 2020 9:41 pm
Re: Textbook Problem 1B.15
for part b use einstein's equation (e = hv). V is given and h is Heisenberg's constant.
Return to “Photoelectric Effect”
Who is online
Users browsing this forum: No registered users and 11 guests

