Work Function Question
Moderators: Chem_Mod, Chem_Admin
-
Catherine Bubser 2C
- Posts: 114
- Joined: Wed Sep 30, 2020 9:45 pm
Work Function Question
When solving for kinetic energy, will the work function be added or subtracted based on if absorption or emission is occurring or is it always E= hv - work?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Work Function Question
You use the work function with photoelectric problems, not with absorption/emission spectra. The equation for photoelectric problems is
energy of incoming light-work function= kinetic energy
energy of incoming light-work function= kinetic energy
-
Shivani Sakthi 1l
- Posts: 102
- Joined: Fri Sep 24, 2021 7:02 am
- Been upvoted: 1 time
Re: Work Function Question
The work function equation concerns the photoelectric effect which functions under the following principles:
1. no electrons can be ejected unless the initial energy of the radiation has a frequency above the threshold energy, or minimum energy required to eject electrons from metal.
2. regardless of how low intensity the radiation is, electrons are ejected immediately.
3. the kinetic energy of electrons that are ejected increases linearly with the frequency of the incident radiation
4. if the energy of the photon is less than the threshold energy, the electron is not ejected regardless of it's intensity. However, if the energy of the photon is greater than the threshold energy/work function, electrons are ejected with kinetic energy.
Taking into account these underlying principles, the equation becomes Energy of photon or hV= threshold energy/workfunction + kinetic energy.
So when solving for kinetic energy, the work function will be subtracted from the energy of the photon. If it helps, the kinetic energy equation is also KE=1/2 mv^2 . I hope this helped!
1. no electrons can be ejected unless the initial energy of the radiation has a frequency above the threshold energy, or minimum energy required to eject electrons from metal.
2. regardless of how low intensity the radiation is, electrons are ejected immediately.
3. the kinetic energy of electrons that are ejected increases linearly with the frequency of the incident radiation
4. if the energy of the photon is less than the threshold energy, the electron is not ejected regardless of it's intensity. However, if the energy of the photon is greater than the threshold energy/work function, electrons are ejected with kinetic energy.
Taking into account these underlying principles, the equation becomes Energy of photon or hV= threshold energy/workfunction + kinetic energy.
So when solving for kinetic energy, the work function will be subtracted from the energy of the photon. If it helps, the kinetic energy equation is also KE=1/2 mv^2 . I hope this helped!
-
Sarah Hong 2K
- Posts: 102
- Joined: Fri Sep 24, 2021 5:48 am
Re: Work Function Question
When solving for kinetic energy, you will subtract the work function (threshold energy) to the energy of the photon (E=hV).
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Work Function Question
Hello Catherine,
Please refer to the following equality for all photoelectric scenarios: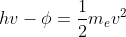 .
.
Please refer to the following equality for all photoelectric scenarios:
Return to “Photoelectric Effect”
Who is online
Users browsing this forum: No registered users and 3 guests

