Hi, I had a question about the achieve homework for week 2. The question is:
Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level n=7
to the level n=1.
I understand that you have to use the En = - (hR)/(n^2) formula but I am not sure what to do after this step. For E7 I got -4.45x10^-20 J and for E1 I got -2.18 x 10^-18 J.
Achieve Week 2 Question 8
Moderators: Chem_Mod, Chem_Admin
-
Brian Huynh 2L
- Posts: 180
- Joined: Fri Sep 29, 2023 11:01 am
- Been upvoted: 2 times
Re: Achieve Week 2 Question 8
The energy (E) of the photon emitted is equivalent to the difference between the energy levels, therefore, Efinal - Einitial = E. And because E = hv (Planck's Constant x frequency) and c(speed of light) = lambda (wavelength) x v (frequency) we can find the relationship between the energy of the photon it's wave length;
Efinal - Einitial = hv
c= (lambda)(v)
rearrange:
v = c / lambda
Plug in c/lambda to represent v in the equation:
Efinal - Einitial = hc/ lambda
Isolate lambda:
lambda (Efinal - Einitial) = hc
lambda = hc / (Efinal - Einitial)
Efinal; -(hR)/(1^2)
Einitial; -(hR)/(7^2)
You should get a negative number, but remember the energy of a photon cannot be negative; this number is negative because when an eletron transitions to a lower energy level it loses energy by emitting it as a photon (here we are calculating how much energy an electron loses when transition between n=7 to n=1). Therefore, the energy of the photon will be the absolute value of the number you get.
Efinal - Einitial = hv
c= (lambda)(v)
rearrange:
v = c / lambda
Plug in c/lambda to represent v in the equation:
Efinal - Einitial = hc/ lambda
Isolate lambda:
lambda (Efinal - Einitial) = hc
lambda = hc / (Efinal - Einitial)
Efinal; -(hR)/(1^2)
Einitial; -(hR)/(7^2)
You should get a negative number, but remember the energy of a photon cannot be negative; this number is negative because when an eletron transitions to a lower energy level it loses energy by emitting it as a photon (here we are calculating how much energy an electron loses when transition between n=7 to n=1). Therefore, the energy of the photon will be the absolute value of the number you get.
-
Erika Patel 3I
- Posts: 106
- Joined: Fri Sep 29, 2023 11:03 am
Re: Achieve Week 2 Question 8
Yes, you are on the right track! From there you need to find the change in energy (Efinal-Einitial -- i.e. E1-E7) when going from energy level n=7 to n=1. Then you can combine the equations E=hv and c =  v to form
v to form 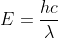 . Rearrange for wavelength, plug in your energy value, planck's constant and speed of light and you will get wavelength. Then convert to nm.
. Rearrange for wavelength, plug in your energy value, planck's constant and speed of light and you will get wavelength. Then convert to nm.
-
Joseph Fisher 2K
- Posts: 82
- Joined: Fri Sep 29, 2023 10:58 am
Re: Achieve Week 2 Question 8
You can use this equation to solve it: 1/λ = (1.0974 x 10^7)(1/(n1)^2 - 1/(n2)^2)
With this equation, you should be able to plug in your numbers and solve for λ. But make sure to note units and the request for the answer in nm.
With this equation, you should be able to plug in your numbers and solve for λ. But make sure to note units and the request for the answer in nm.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 2 guests

