Bohr Frequency Condition
Moderators: Chem_Mod, Chem_Admin
-
Stephanie Torres 2F
- Posts: 37
- Joined: Fri Sep 29, 2023 10:59 am
Bohr Frequency Condition
Can someone elaborate on what specifically the Bohr frequency condition is and under what terms it is used?
-
jasi_bermejo_3h
- Posts: 94
- Joined: Fri Sep 29, 2023 11:42 am
Re: Bohr Frequency Condition
I believe that the Bohr Frequency Condition, delta E = hv, is of the idea that when the frequency of incoming light matches the energy difference for the transition of electrons between energy levels, you will have absorption of light.
-
Joyce Meng 2J
- Posts: 83
- Joined: Fri Sep 29, 2023 11:04 am
Re: Bohr Frequency Condition
The Bohr Frequency Condition is a formula saying frequency = change in energy/Planck's constant, or 
This formula means that for every frequency of an element, there needs to be a very specific change in energy when an atom absorbs or emits energy to make it to another energy level. For example, when a hydrogen atom emits energy so that it goes from n=4 to n=3, the frequency that it emits will always be the same, so the change in energy will always be the same from n=4 to n=3. This is what gives each element its own unique "fingerprint".
This formula is used for atomic spectra problems and is often paired with the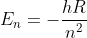 to create the Rydberg equation.
to create the Rydberg equation.
This formula means that for every frequency of an element, there needs to be a very specific change in energy when an atom absorbs or emits energy to make it to another energy level. For example, when a hydrogen atom emits energy so that it goes from n=4 to n=3, the frequency that it emits will always be the same, so the change in energy will always be the same from n=4 to n=3. This is what gives each element its own unique "fingerprint".
This formula is used for atomic spectra problems and is often paired with the
-
Kyle_Phong_2J
- Posts: 110
- Joined: Fri Sep 29, 2023 11:30 am
Re: Bohr Frequency Condition
Bohr's frequency condition is the equation E = hν where E is the energy difference when an electron goes from a higher level to a lower level of energy (like n=4 to n=1), h is Planck's constant, and v is the frequency of the photon. We use this equation when trying to find frequency or switching variables around to find wavelength.
Ex) We have these two equations: E=hv and c=λv
If we are trying to solve for wavelength, we rearrange variables to get v=c/λ, E=hc/λ, and finally λ=hc/E
Ex) We have these two equations: E=hv and c=λv
If we are trying to solve for wavelength, we rearrange variables to get v=c/λ, E=hc/λ, and finally λ=hc/E
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 2 guests

