Hi, I have two questions:
1. when we are finding the change in energy using the equations, are we finding the change in energy of only the electron? Will the change in energy be either positive or negative based on if the electron is absorbing or emitting?
2. When an electron emits a photon, will the energy of the photon always be labeled as positive?
Change in energy of an electron [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Nishma Chakraborty 1J
- Posts: 51
- Joined: Fri Sep 29, 2017 7:04 am
Re: Change in energy of an electron
Hey!
For 2) I'm pretty sure that when an electron emits a photon, the value of energy will be a positive number. Honestly, I'm not too sure about 1) myself, so if anyone else could clarify, that'd be great! Thanks!
For 2) I'm pretty sure that when an electron emits a photon, the value of energy will be a positive number. Honestly, I'm not too sure about 1) myself, so if anyone else could clarify, that'd be great! Thanks!
-
Nancy Le - 1F
- Posts: 52
- Joined: Fri Sep 29, 2017 7:07 am
Re: Change in energy of an electron
The positive and negative signs only indicate whether the photon is emitted or absorbed, but the amount of energy that is used is always positive.
Hope this helps!
Hope this helps!
-
Leanne Wong 1H
- Posts: 50
- Joined: Tue Oct 10, 2017 7:13 am
- Been upvoted: 1 time
Re: Change in energy of an electron [ENDORSED]
When we're solving for the change in energy, it gives us the energy emitted by the electrons because of E (photon) - E (energy remove e-) = 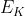 which is the energy of the electron emitted.
which is the energy of the electron emitted.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 3 guests

