Hi!
For quiz 2 if it asks us for example to calculate the frequency of light emitted by an electron that goes from n=4 to n=2 can I use the Rydberg equation:
frequency = R [(1/n1^1) - (1/n2^1)]
or should I go through these calculations:
delta E = E(final) - E(initial) = E2-E4= (-1/4*h*R) + (1/16*h*R) and then find the frequency by doing frequency=delta E/h
Thank you so much!
Anna De Schutter - section 1A
Quiz 2 [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
Re: Quiz 2
I asked some of the TAs/UAs and they said that it would be fine to use the Rydberg equation, because essentially the Rydberg formula is just a simplified equation derived from 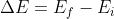 (where
(where 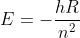 ). I believe the purpose of Dr. Lavelle doing it step-by-step in class was to show us how calculating energy in transition states makes sense conceptually; rather than just giving us a formula and accepting it as true.
). I believe the purpose of Dr. Lavelle doing it step-by-step in class was to show us how calculating energy in transition states makes sense conceptually; rather than just giving us a formula and accepting it as true.
-
Tina Wen 1G
- Posts: 31
- Joined: Fri Apr 06, 2018 11:05 am
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Quiz 2 [ENDORSED]
What do you mean by the original equation? You are expected to conceptually understand the equation for energy given you to by Prof. Lavelle in class. He prefers this over the Rydberg equation, because that equation allows you to plug and chug without understanding the idea behind it.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 5 guests

