Help?? Im confused as to how we are supposed to do this since we are given the wavelength.
Test 2 Q.4
Moderators: Chem_Mod, Chem_Admin
-
Andrea Raymundo 1B
- Posts: 32
- Joined: Wed Nov 15, 2017 3:04 am
Test 2 Q.4
Q: A cotton ball soaked in 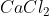 is lit on fire. The flame is a dark orange color
is lit on fire. The flame is a dark orange color ) . A scientist claims that the color arises from the transition of an electron from its excited state to its ground state. What is the difference in energy between these two states?
. A scientist claims that the color arises from the transition of an electron from its excited state to its ground state. What is the difference in energy between these two states?
Help?? Im confused as to how we are supposed to do this since we are given the wavelength.
Help?? Im confused as to how we are supposed to do this since we are given the wavelength.
-
AnnaYan_1l
- Posts: 96
- Joined: Fri Apr 06, 2018 11:05 am
- Been upvoted: 1 time
Re: Test 2 Q.4
You can use the wavelength to determine the energy released (where an electron went from a higher energy level to a lower energy level). This energy is the difference in energy between the two states.
Hope this helps! Try to work out the problem, and if you are still having trouble I can write out the steps.
Hope this helps! Try to work out the problem, and if you are still having trouble I can write out the steps.
-
Yadira Flores 1G
- Posts: 41
- Joined: Wed Nov 15, 2017 3:01 am
Re: Test 2 Q.4
I have the problem worked out, would it be possible for anyone to work out the steps? Just want to make sure I did it correctly.
-
madisonhanson1b
- Posts: 30
- Joined: Fri Apr 06, 2018 11:03 am
Re: Test 2 Q.4
You can use two equations to reach a final answer. The first equation is deltaE=hv(frequency). You can then use the equation v(frequency)=c/wavelength as a substitute for v(frequency). This will give you the combined equation of deltaE=hc/wavelength. H and C are both constants and the wavelength is given to you.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 9 guests

