Why is there a negative?
Moderators: Chem_Mod, Chem_Admin
-
Ethan Breaux 2F
- Posts: 63
- Joined: Sat Sep 29, 2018 12:16 am
Why is there a negative?
Why is there a negative in the equation (En = -hR/n^2) when we just put the positive value whenever a questions asks us to find the frequency or wavelength? Is there any scenario in which we would put the negative value as the answer?
-
gillianozawa4I
- Posts: 31
- Joined: Fri Sep 28, 2018 12:27 am
Re: Why is there a negative?
I think there is a negative sign in the equation to show that energy is emitted (because the electron goes from a higher to a lower energy state). If energy was absorbed, the answer would end up being positive. Hope this helps!
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Why is there a negative?
The reason why there's a negative in the equation you listed is because energy levels closer to the nucleus are relative to the theoretically highest-most energy level that is an infinite distance away from the nucleus. The energy level of the n=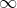 level is zero, since there's no attraction between the nucleus and any electrons in that level. Because energy levels closer to the nucleus are lower in energy than energy levels farther away from the nucleus, anything below the n=
level is zero, since there's no attraction between the nucleus and any electrons in that level. Because energy levels closer to the nucleus are lower in energy than energy levels farther away from the nucleus, anything below the n=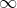 level must be negative (since less than zero). This is why the equation is negative. The reason you only use the positive magnitude when calculating the frequency or wavelength of light from the energy is because the energy that an electron loses from a higher energy to a lower energy is released in the form of light. Due to law of conservation of energy, the energy that the electron loses is released in the form of light. Energy of light can't ever be negative, but the change in energy of an electron can be negative (lost energy from perspective of electron).
level must be negative (since less than zero). This is why the equation is negative. The reason you only use the positive magnitude when calculating the frequency or wavelength of light from the energy is because the energy that an electron loses from a higher energy to a lower energy is released in the form of light. Due to law of conservation of energy, the energy that the electron loses is released in the form of light. Energy of light can't ever be negative, but the change in energy of an electron can be negative (lost energy from perspective of electron).
-
mayra martinez 1D
- Posts: 32
- Joined: Fri Sep 28, 2018 12:17 am
Re: Why is there a negative?
Yea so to reiterate, as the n increases, the energies of successive levels increase (thus become less negative) until you approach zero, where the electron is on the point of escaping the atom. All the energies are negative because the electron has a lower energy in the atom, than when its far from the nucleus.
-
g orloff 1J
- Posts: 45
- Joined: Fri Sep 28, 2018 12:16 am
Re: Why is there a negative?
to think about it in simpler terms, energy is taken out/ removed from the system therefore there is a negative.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 11 guests

