When to use the Bohr Frequency Condition?
Moderators: Chem_Mod, Chem_Admin
-
Kimberly Bauer 4E
- Posts: 51
- Joined: Thu Jul 25, 2019 12:17 am
When to use the Bohr Frequency Condition?
When do we use the Bohr Frequency Condition and what is the background on it?
-
Julie Park 1G
- Posts: 100
- Joined: Thu Jul 25, 2019 12:15 am
Re: When to use the Bohr Frequency Condition?
You would use it to describe the relation between the change in energy of an atom (as it transitions between two states) or molecule & the frequency of radiation emitted or absorbed.
The equation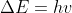 or
or 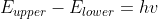 describes this relation.
describes this relation.
The equation
-
RasikaObla_4I
- Posts: 100
- Joined: Thu Jul 25, 2019 12:15 am
Re: When to use the Bohr Frequency Condition?
The Bohr Frequency Condition is used when a high energy electron drops to a lower energy level and the difference in energy is given off as a photon.
-
Giselle Littleton 1F
- Posts: 78
- Joined: Wed Sep 18, 2019 12:20 am
Re: When to use the Bohr Frequency Condition?
The Bohr frequency condition demonstrates an electron's transition between energy levels. Differences in energy levels are given off as photons and only photons of certain energy are given off exactly matching the energy level difference.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 5 guests

