Rydberg's Constant
Moderators: Chem_Mod, Chem_Admin
Rydberg's Constant
For Rydberg's constant, we should only use the one provided in the equations and constants sheet Dr. Lavelle has on his website, right? And we should stick to E=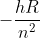 rather than Rydberg's equation?
rather than Rydberg's equation?
-
Naneeta Desar 1K
- Posts: 106
- Joined: Fri Aug 09, 2019 12:15 am
-
JTijerina_4A
- Posts: 46
- Joined: Mon Sep 23, 2019 12:17 am
Re: Rydberg's Constant
Rydberg's can be derived from the equations given to us. So that we can connect it to what we are learning conceptually, Lavelle wants us to be able to manipulate the given equation rather than memorizing Rydberg's.
Re: Rydberg's Constant
Does anyone know what the actual constant is for Rydberg's constant? Or is it the (1/n1 - 1/n2)?
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 2 guests

