I was trying to calculate the energy of light absorbed from an electron (in a hydrogen atom) going from n=2 to n=4.
In order to solve it I used the equation
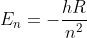
and plugged in n=2 and then n=4 and then used plugged those results into the deltaE = Efinal - Einitial (deltaE = E(4) - E(2)). When I did that I did not get the right answer so then I tried using the Rydberg equation with n1=4 and n2=2 (
)
) and then used plugged the answer I got from that into E=hv, and that gave me the right answer.
I thought that either way would give you the same answer because I thought that
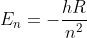
would give me the energy at certain n levels and then I could subtract them to get the amount absorbed. Why doesn't that equation work but the Rydberg equation does?