The electron in a hydrogen atom is excited to the n=7 shell and emits electromagnetic radiation when returning to lower energy levels. Determine the number of spectral lines that could appear when this electron returns to the lower energy levels, as well as the wavelength range in nanometers.
What I did for the second part:
- I used the equation, v=Rh(1/n^2(lower energy level)-1/n^2(higher energy level)). I used this equation to find the difference between n=7 to n=6 and then n=7 to n=1.
I got 1.24*10^-5 and then 9.30*10^-8
I was wondering what I should do next to find the wavelength range. (also what would the units be for the answer I found above because I am trying to practice keeping track of that)
Thanks!:)
Question 10 from Homework week 2,3,4
Moderators: Chem_Mod, Chem_Admin
-
Zinnia Kwan 3D
- Posts: 61
- Joined: Fri Sep 24, 2021 5:49 am
- Been upvoted: 2 times
Re: Question 10 from Homework week 2,3,4
The next step would be to convert from frequency to wavelength using the formula C= (wavelength) v, or wavelength = C/v, using the two frequencies you calculated earlier. Afterwards, you should get wavelength in meters, but the question asks for nanometers so we would multiply by the stoichiometric conversion: 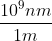 to get our answers.
to get our answers.
(also, in my achieve, it uses n=6 shell in the problem but maybe mine is just different. Hope this helps!)
(also, in my achieve, it uses n=6 shell in the problem but maybe mine is just different. Hope this helps!)
-
Brandon Achugbue 3H
- Posts: 66
- Joined: Fri Sep 24, 2021 5:16 am
Re: Question 10 from Homework week 2,3,4
I'm pretty sure by wavelength range they're asking for the shortest and longest wavelengths which you've already calculated. Just remember the longer jump in energy level = more energy produced = shorter wavelength, so the wavelength you calculate that corresponds to n = 7 to n= 1 should be the shorter length, and the one that corresponds to n = 7 to n = 6 would be the longer one. (*I think)
-
Nithya Madhu 2L
- Posts: 100
- Joined: Fri Sep 24, 2021 6:28 am
Re: Question 10 from Homework week 2,3,4
Hey, I think our problems are different but the next step is just to find the wavelengths by using lamda = c/v. Also remember to convert your frequency from m to nm by multiplying it by 10^9.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 6 guests

