Bohr Frequency Condition
Moderators: Chem_Mod, Chem_Admin
-
Carly Yoon
- Posts: 79
- Joined: Fri Sep 24, 2021 7:30 am
Bohr Frequency Condition
I am confused about the Bohr Frequency Condition and what it refers to. What do we need to know regarding this topic?
Re: Bohr Frequency Condition
From my understanding, the most important takeaway from the Bohr Frequency Condition is that an electron will be excited if and only if the energy of the incoming photon exactly matches the energy difference. This condition is summarized by the equation 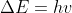 .
.
Re: Bohr Frequency Condition
The Bohr frequency condition is the frequency absorbed or emitted during the transition of an electron between energy levels. The Bohr frequency condition can be represented by the equation: v = (delta)E / h , where v represents frequency, (delta)E represents the difference in energy, and h represents Planck's constant.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 5 guests

